PUTTING AMR ACCELERATOR SCIENCE ON THE COMMUNITY AGENDA
In connection with the AMR Conference in Basel this year, all nine AMR Accelerator projects met to discuss progress, collaboration, and sustainability. During the conference, experts from several AMR Accelerator projects contributed to the discussion in presentations and panels, putting some of the challenges for antibiotics development on the agenda for the AMR community.

After a first successful face-to-face AMR Accelerator cross-project meeting hosted last year by COMBINE, the partners were eagerly waiting to meet again in 2023 to exchange on the expected project results and explore their potential impact on the AMR research community and society. This year, all nine AMR Accelerator projects (AB-Direct, COMBINE, ERA4TB, GNA NOW, PrIMAVeRa, RespiriNTM, RespiriTB, TRIC-TB, UNITE4TB) were represented by more than 40 participants who had the chance to listen to project pitches showcasing the progression of the research assets, and also anticipating their sustainability and exploitability post-project. By the time the AMR Accelerator meets again next year, two of the nine projects will have come to an end: TRIC-TB and AB-Direct. Translating project research into direct outcomes was thus high on this year’s meeting agenda. To support this goal, COMBINE has put together three Scientific Interest Groups for the AMR Accelerator, aiming to create synergies between partners. During the cross-project meeting, the groups discussed:

- community engagement, with the remote participation of Patrick Agbassi, Global TB Community Advisory Board and UNITE4TB Community Advisory Group, and Andy Gibson, University of West England and People in Health West of England;
- the application of machine learning to antibiotic drug discovery, with a hackathon on how to leverage existing data for pattern identification;
- the standardisation of animal models of values for antimicrobial resistance (AMR), with the presentation of a new data catalog of AMR models used for Gram-negative infections, among others.

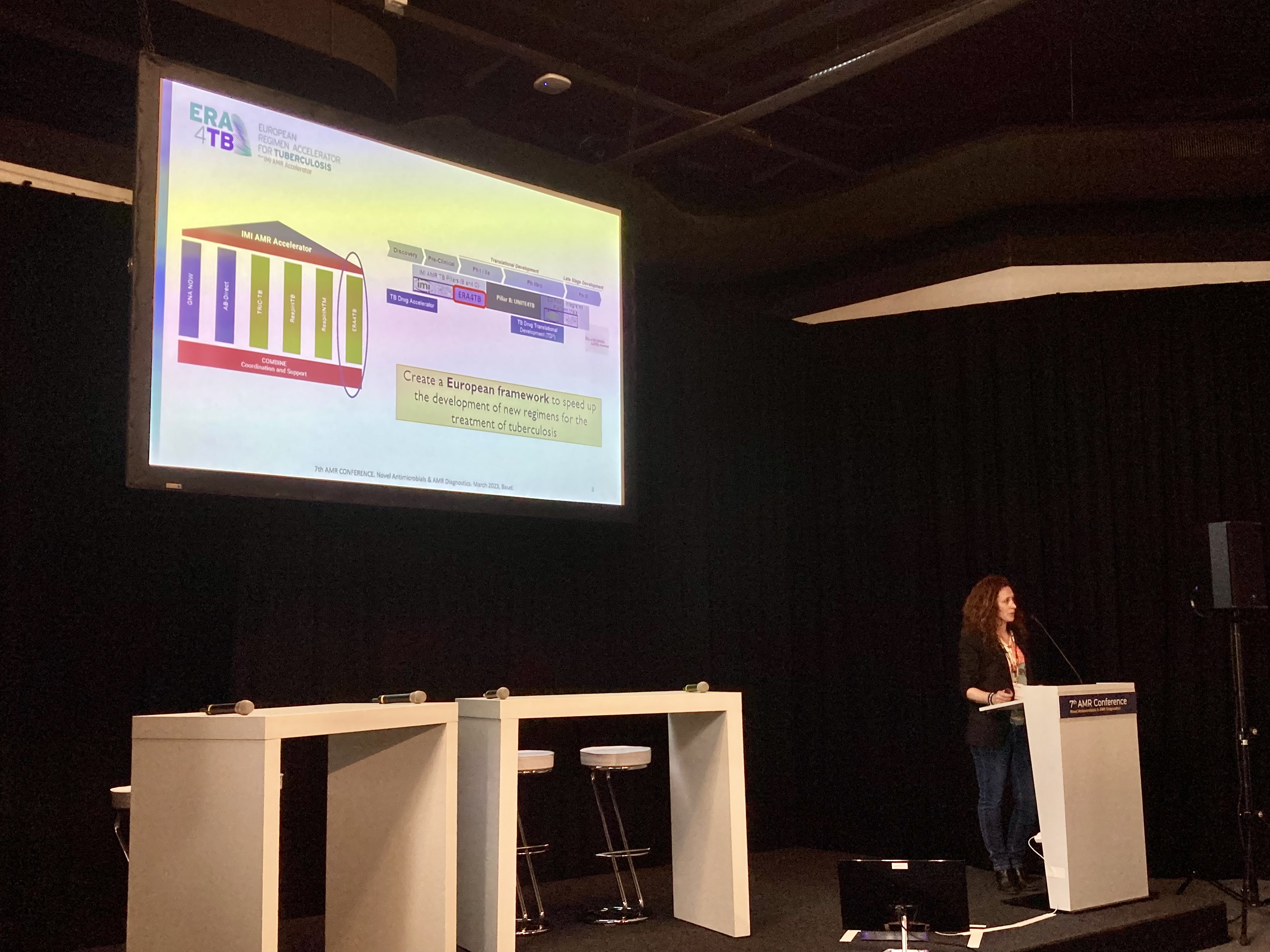
On the following two days, the AMR Accelerator was represented at the AMR Conference alongside global players in the field, such as the Global Antibiotic Research & Development Partnership (GARDP), the Global AMR R&D Hub, and the Incubator for Antibacterial Therapies in Europe (INCATE), with an exhibition booth, showcasing more than 15 short video clips about the projects as well as print materials. Several project partners also presented and discussed insights from their research in various sessions and posters. On the first day, COMBINE members from Paul-Ehrlich-Institut moderated a panel on advancing the biological pipeline against AMR pathogens and provided a snapshot of the project’s work on standardising the murine pneumonia model in a session highlighting novel tools in pre-clinical development. On the second day, UNITE4TB and ERA4TB members, Martin Boeree, Radboud University, Santiago Ferrer Bazaga, Universidad Carlos III de Madrid, and Ainhoa Lucia Quintana, University of Zaragoza, shared their experiences with the audience on treatment combinations against tuberculosis. Other partners involved in the conference programme included GNA NOW member Shampa Das, University of Liverpool, Anders Karlén, COMBINE Coordinator, Uppsala University, and Marlieke De Kraker, University of Geneva, who presented the ECRAID project, among others, and is also leading PrIMAVeRa project’s efforts in assessing the availability and quality of published evidence about the burden of drug-resistant bacterial infections in a healthcare context.
You can find more impressions from these three days in this photo library:
About the AMR Accelerator
The aim of the Antimicrobial Resistance (AMR) Accelerator Programme is to progress the development of new medicines to treat or even prevent resistant bacterial infections in Europe and worldwide. The programme comprises the following three pillars: a Capability Building Network, a Tuberculosis Drug Development Network, and the Portfolio Building Networks. The scope of the AMR Accelerator is broad; under one structure, it addresses many of the scientific challenges of AMR, and it supports the development of new ways to prevent and treat AMR. More broadly, the AMR Accelerator contributes to the European action plan on AMR.
For more information on the AMR Accelerator, please visit https://www.amr-accelerator.eu/
About COMBINE
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 853967. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution.
About the Innovative Medicine Initiative
About the Innovative Medicine Initiative The Innovative Medicines Initiative (IMI) is Europe’s largest public-private initiative aiming to speed up the development of better and safer medicines for patients. IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. IMI is a joint undertaking between the European Union and the European Federation of Pharmaceutical Industries and Associations, EFPIA.
For more information on IMI, please visit https://www.imi.europa.eu/
Disclaimer
This communication reflects the views of the authors and neither IMI nor the European Union and EFPIA are liable for any use that may be made of the information contained herein.