Follow us:
Mission and Vision
ERA4TB (European Accelerator of Tuberculosis Regime) project is a public-private initiative devoted to accelerate the development of new treatment regimens for tuberculosis.
ERA4TB is expected to revolutionize the way in which tuberculosis treatments are developed thanks to its parallelized, multi-entry pipeline structure, analogue to a production line. This structure will enable to systematically investigate the efficacy of several drug candidates and combinations simultaneously while allowing new molecules to enter the project pipeline at the research stage corresponding to the degree of knowledge on said candidate drugs gathered before the project.
With this approach, the ERA4TB consortium expects to reduce the time required for the development of new tuberculosis treatment regimens by up to a quarter.
The ERA4TB initiative integrates more than thirty organizations from the European Union and the United States among which are the main global actors in the fight against tuberculosis infection.
ERA4TB has started in 2020 and will last six years, at the end of which, the consortium expects to have developed at least two or more new combination regimens with treatment-shortening potential ready for Phase II clinical evaluation. The partners intend to maintain the ERA4TB platform active beyond the project official duration.
Strategy
The main objective of ERA4TB is to create a European open platform to accelerate the development of new regimens for the treatment of tuberculosis. To reach this goal, the consortium has set the following specific objectives:
- Implementation of state-of-the-art tools and capacities into an open platform for the evaluation of TB drug candidates to effectively progress compounds from early preclinical to clinical development and identify potential new Pan-tuberculosis (Pan-TB)1 regimens ready for Phase II clinical evaluation.
- Development of modelling and simulation tools and application of standard and new artificial intelligence (AI) techniques for better characterization of pharmacokinetic-pharmacodynamic (PK/PD) relationships (concentration-effect or exposure-response relationships depending on the trial), optimization of clinical trial design, prediction of therapeutic dose range and antibacterial activity in humans.
- Management of data generated by the project, integrating also data and knowledge from historical datasets available in reference databases and from previous and existing consortia and projects, in the context of an ever-improving ‘learning system’ that allows to refine the platform continuously.
- Provision of a flexible and efficient management structure able to adapt to the capacity and resource allocation level required by each platform component at each stage of the project, depending on each compound’s progression and attrition dynamics and on inherent variables of the multiple combination assays.
- Provide a sustainability plan that incorporates all the synergies and lessons learned within the project and secures the survival of the platform beyond the life of the project.
- Define and execute an outreach, engagement, dissemination and communication plan in collaboration with regulatory authorities and other stakeholders, including patient organizations, to maximize the impact of the project.
All Member States of WHO and the UN have committed to the goal of ending the TB epidemic by 2030 through their unanimous endorsement of WHO’s End TB Strategy at the World Health Assembly and their adoption of the UN Sustainable Development Goals (SDGs). The commitment of the European Commission and the private sector (through the EFPIA companies), with the support of Associated Partners as key players in TB research, as well as other public and private research institutions in the EU to respond to this global challenge is materialised through the ERA4TB Consortium.
ERA4TB aims to build across Europe and consolidate globally a solid network of collaborations with the ambition of creating a novel world-class platform for the effective acceleration of new anti-TB drugs and regimens. This new platform, that intends to operate beyond the life of this project, will integrate, maintain and further advance the drug development processes and tools (including cutting edge solutions such as hollow fibre systems, single cell time-lapse analysis, imaging in animal models, new biomarkers and host/pathogen interactions and virulence approaches, drug-disease modelling, physiologically-based predictive modelling, artificial intelligence data mining and clinical trial simulation techniques) needed for the effective acceleration of anti-TB drug combinations.
The open philosophy of the platform, both in terms of capacity (by enabling participation of all relevant research groups and actors) and tools (by designing a pipeline that allows the ‘plugging-in’ of new promising techniques and assays as they become available) is expected to underpin long-termsustainability and position ERA4TB as a truly unique programme that leverages the best available knowledge and expertise in Europe. Here, we are inspired by precedents in other scientific areas, such as the CERN in physics, where a vibrant and competitive European community exists.
Governance
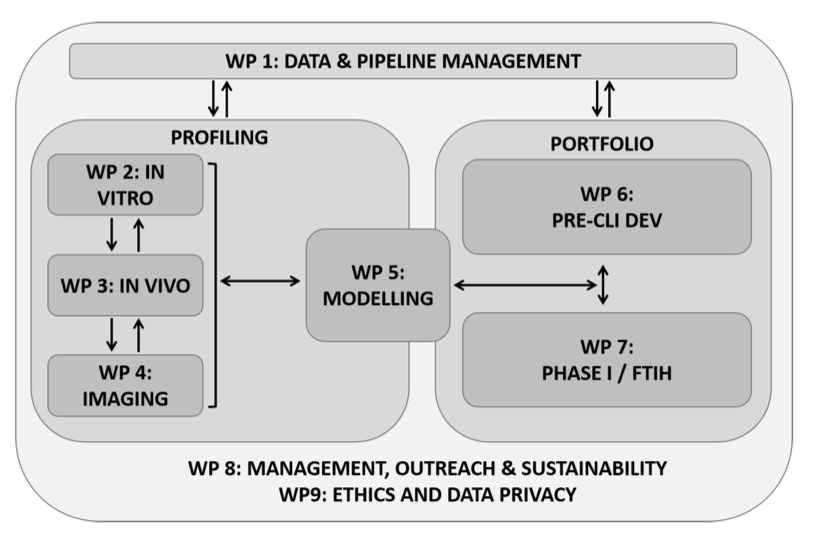
The ERA4TB platform is based on a progression pipeline that can cater for a variety of molecules at different stages of development. The drug candidates entering the pipeline will be supplied by EFPIA and Associated Partners and progress through the required research phases until reaching Phase I FTIH (First Time in Humans) clinical trials.
It is the mission of the consortium to bring to Phase I the maximum number of molecules suitable for inclusion in new anti-TB regimens. In view of this, the project’s development activities have been divided into two main integrated areas: Preclinical Profilingand Portfolio Development, the latest including pharma Development and Phase I FTIH. The separation of these activities allows the Consortium to develop a comprehensive work plan that accommodates the different entry points for the various molecules, ensuring flexibility to identify the expertise required and adjust resource allocation and workload distribution appropriately.
Publications
- Aguilar-Ayala D. A., Sanz-García F., Rabodoarivelo M. S., Susanto B. O., Bailo R., Eveque-Mourroux M. R., Willand N., Simonsson U. S. H., Ramón-García S., Lucía A. (2024) Evaluation of critical parameters in the hollow-fibre system for tuberculosis: A case study of moxifloxacin, British Journal of Clinical Pharmacology, DOI: 10.1111/bcp.16068
- Nagar, S., Nicholls, D., Dawoud, D. (2024) A systematic review of economic evaluations of pharmacological treatments for active tuberculosis, Frontiers in Public Health, DOI: 10.3389/fpubh.2024.1201512
- Moraga, P., Prieto, P., Conradie, A., Benhayoun, M., Rousell, V., Davy, M., Fuhr, U., Arbos, R. A., Abad-Santos, F., Portolés, A., Van Duinen, J., Carcas, A. J., Borobia, A. M. (2023) Academia and industry agreement on a feasibility tool for first-time-in-human clinical trial units, American Society for Clinical Pharmacology & Therapeutics, Clinical and Translational Science, DOI: 10.1111/cts.13655
- van Wijk, R. C., Lucía, A., Sudhakar, P. K., Sonnenkalb, L., Gaudin, C., Hoffmann, E., Dremierre, B., Aguilar-Ayala, D. A., Dal Molin, M., Rybniker, J., de Giorgi, S., Cioetto-Mazzabò, L., Segafreddo, G., Manganelli, R., Degiacomi, G., Recchia, D., Pasca, M. R., Simonsson, U., Ramón-García, S. (2023) Implementing best practices on data generation and reporting of Mycobacterium tuberculosis in vitro assays within the ERA4TB consortium, CellPress, iScience, DOI: 10.1016/j.isci.2023.106411
- Mistretta, M., Gangneux, N., Manina, G. (2022) Microfluidic dose–response platform to track the dynamics of drug response in single mycobacterial cells, Nature, Scientific Reports, DOI: 10.1038/s41598-022-24175-9
- Thacker, V. V., Dhar, N., Sharma, K., Barrile, R., Karalis, K., McKinney, J. D. (2020) A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth, eLife, Microbiology and Infectious Disease, Physics of Living Systems, DOI: 10.7554/eLife.59961
- Faraj, A., Clewe, O., Svensson, R. J., Mukamolova, G. V., Barer M. R., Simonsson, U. (2020) Difference in persistent tuberculosis bacteria between in vitro and sputum from patients: implications for translational predictions, Nature, Scientific Reports, DOI: 10.1038/s41598-020-72472-y
Project consortium
Partners
The ERA4TB Consortium brings together a multi-disciplinary team with proven expertise and capabilities in TB drug development to profile and progress anti-TB compounds up to completion of Phase I.
The project consortium integrates 31 organizations, namely eight prestigious academic institutions (UC3M, UNIZAR, UU, EPFL, UKÖ, UNIPD, UPV, LUND), four non-profit organizations (IPP, IPL, iM4TB, BAR), eight public research organizations (FZB, CNR, IDMIT, SERMAS, PHE, NICE, SCI, IOS) and five highly skilled small-medium enterprises (SYNAPSE, C-Path, IBT, QPS, GRIT), together with three EFPIA members (GSK, EVT, JANSSEN), and three IMI2 Associated Partners (BMGF, TBA, DDU).
| Logo | Short name | Partners full name | Link |
|---|---|---|---|
 |
UC3M | University Carlos III Madrid | https://www.uc3m.es/Home |
 |
IPP | Institute Pasteur (Paris) | https://www.pasteur.fr/en/institut-pasteur |
 |
UNIZAR | University of Zaragoza / ARAID | www.unizar.es |
 |
IPL | Institute Pasteur de Lille Foundation | https://www.pasteur-lille.fr/5/research/units/ |
 |
SYNAPSE | Synapse Managers Partners | https://synapse-managers.com/ |
 |
BOR | Forschungszentrum Borstel | https://fz-borstel.de/index.php/de/ |
 |
IM4TB | Foundation Innovative Medicines for Tuberculosis | http://im4tb.org |
 |
C-Path | Critical Path Institute, Limited | https://c-path.eu/ |
 |
CNR | Consiglio Nazionale delle Richerche | https://www.iac.cnr.it/ |
 |
IDMIT | Infectious Diseases Models for Innovative Therapies | https://idmitcenter.fr/symposium/ |
 |
SERMAS | Instituto de Investigación Hospital Universitario La Paz | http://www.idipaz.es |
 |
UU | Uppsala University | https://www.uu.se/en |
 |
PHE | Public Health England- Department of Health | https://www.gov.uk/government/organisations/public-health-england/about |
 |
EPFL | École polytechnique fédérale de Lausanne | https://www.epfl.ch/labs/mckinney-lab/ |
 |
UKÖ | University of Köln | http://www.uni-koeln.de |
 |
UNIPD | University of Padova | https://www.unipd.it/en/ |
 |
UPV | University of Pavia | https://web.unipv.it |
 |
NICE | The National Institute for Health and Care Excellence | https://www.nice.org.uk/about |
 |
IBT | ImaBiotech | www.imabiotech.com |
 |
SCI | Sciensano | https://www.sciensano.be/en/about-sciensano/sciensanos-organogram/bacterial-diseases/tuberculosis-and-mycobacteria#want-to-know-more- |
 |
LIOS | Latvia Institute of Organic Synthesis | http://www.osi.lv/en/. |
 |
BAR | Bioaster Foundation de Coopération Scientifique | https://www.bioaster.org/bioaster/bioaster-community/ |
 |
QPS | QPS Netherlands BV | https://www.qps.com |
 |
LUND | Phase I Unit of Skane Hospital | https://sodrasjukvardsregionen.se/clinicaltrialunit/ |
 |
GSK | Glaxosmithkline Investigacion y Desarrollo SL | https://www.gsk.com/ |
 |
EVOTEC | Evotec International GmbH | https://www.evotec.com/en |
 |
Janssen | Janssen Pharmaceutica NV | https://www.janssen.com/ |
 |
BMGF | Bill & Melinda Gates Foundation | https://www.gatesfoundation.org/ |
 |
TBA | Global Alliance for TB Drug Development | https://www.tballiance.org/ |
 |
DDU | University of Dundee | http://www.drugdiscovery.dundee.ac.uk/ |
 |
GRIT42 | Grit systems | https://grit42.com |
 |
CIM – Sant Pau | IIB Sant Pau | http://www.recercasantpau.cat/en/ |
ERA4TB Project Technical Coordinator
Dr. Juan Jose Vaquero
University Carlos III de Madrid
Calle Madrid 126
28 903 Madrid, Spain
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 853989. The JU receives support from the European Union’s Horizon 2020 research and innovation programme, and in-kind support from EFPIA, Global Alliance for TB Drug Development, Bill & Melinda Gates Foundation and University of Dundee. https://www.imi.europa.eu/ |