Mission and Vision
Under the global umbrella of IMI’s AMR Accelerator, the Gram-Negative Antibacterials NOW Consortium (GNA NOW) is a six-and-a-half year project aimed at bringing together key European and private experts in antibiotic discovery and development. The Consortium is committed to developing completely novel compounds derived from previously unknown natural compounds with new modes of action.
Our approach is to leverage the support of a network of platforms with key expertise in the research and development of antibiotics, which is required for a new molecular entity to progress from Lead or pre-candidate/candidate stages up to the completion of Phase I studies. Moreover, GNA NOW aims to contribute significantly to preclinical antibacterial development in the widest sense with capacity and knowledge exchange. To this end, GNA NOW favours the participation of project members in international scientific conferences and topic-related training courses. The project is committed to integrating Patient and Public Involvement (PPI) into compound development and will perform an evaluation of its impact. Finally, the Consortium is developing a quality management system for the use of PK/PD models in preclinical science for better predictions of clinical outcomes which will have clear benefits for the whole AMR community.
Strategy
At the onset, three programmes were run in parallel, each focusing on a different drug candidate with an innovative mode of action. All drug candidates are tested for safety and efficacy to understand and optimise how they work. As with all drug discovery projects, attrition has been anticipated from the onset. GNA NOW applies objective, pre-defined go/no-go criteria in the assessment of ongoing programmes and the recruitment of new ones. So far, two out of the three original programmes reached a no-go decision, resulting in the termination of those programmes within the framework of the GNA NOW project. However, in the summer of 2023, a new programme was adopted by the consortium, continuing the work of testing different drug candidates to enhance the overall pipeline of medicines for patients with infections caused by multidrug-resistant Gram-negative bacteria.
Global Health Infectious Disease (GHID)
There is an urgent unmet need for better antibiotics to combat severe diarrhoea, particularly in the paediatric populations of Low- and Middle-income Countries (LMICs). Currently, there is no vaccine available to prevent these infections and treatment options are decreasing due to resistance emergence and spread of disease. The route for developing drugs against severe bacterial diarrhoea remains unclear.
In this work package (WP7), development compounds, so-called GHID assets, that have shown activity against enteric pathogens Shigella spp., Campylobacter spp., and/or other enterobacteria will be further investigated to assess their suitability for progressing towards Proof-of-Concept clinical studies. As such, the consortium is characterising clinically relevant strains and will develop target product profiles (TPPs) for these diseases.
The ultimate goal of this WP is to generate enough information through the preclinical activities in GNA NOW to fully assess the opportunity of GHID assets to treat severe bacterial enteric infections in LMICs, primarily in paediatric populations, and make a decision on subsequent progression to clinical phases or not. GSK leads WP7.
NOSO-502
NOSO-502 is the first clinical candidate in the novel antibiotic class called Odilhorhabdins that was discovered by Nosopharm. It inhibits the bacterial ribosome with a new mechanism of action and is intended for the treatment of complicated Urinary Tract Infections (cUTI) and complicated Intra-Abdominal Infections (cIAI), including infections caused by polymyxin– and carbapenem-resistant Enterobacteriaceae (CRE). NOSO-502 has proven to be effective in vivo in Enterobacteriaceae infection models and demonstrated antibacterial activity in vitro against multi-drug resistant clinical isolates (NDM- and OXA-expressing strains and KPC among others). In June 2022, an important milestone was reached for the NOSO-502 programme with the completion of the GLP toxicology studies. The results allow for the further development of the programme to Phase 1. Read more in this Consortium press release.
NOSO-2G (terminated)
NOSO-2G is a lead optimisation programme to develop a second-generation Odilorhabdin clinical drug candidate for the treatment of hospital-acquired pneumonia and ventilation-associated pneumonia (HAP/VAP), including infections caused by carbapenem-resistant Pseudomonas aeruginosa. In mid-2022, a decision was made to discontinue the NOSO-2G programme. Despite it showing promising results, more lead optimisation work was needed for which there was a lack of medicinal chemistry resources within GNA NOW. The programme is being further pursued outside of the GNA NOW framework.
Corramycin (terminated)
The natural product Corramycin is a novel scaffold with antibacterial activity against multi resistant Gram-negative pathogens such as Enterobacteriaceae and Acinetobacter Baumannii causing severe hospital bacterial infections (cUTI, and cIAI). Corramycin has no cross resistance with other classes of antibiotics and has a unique mode of penetration into Gram-negative pathogens. In February 2021, a decision was made to discontinue the Corramycin programme due to CMC issues preventing the production of large amounts of the development candidate. Publications on Corramycin are currently being prepared. Following an Open Call in May 2021, the Consortium selected a replacement programme. However, due to budget and timeline reasons the programme was not accepted by the full General Assembly. Other assets are under evaluation.
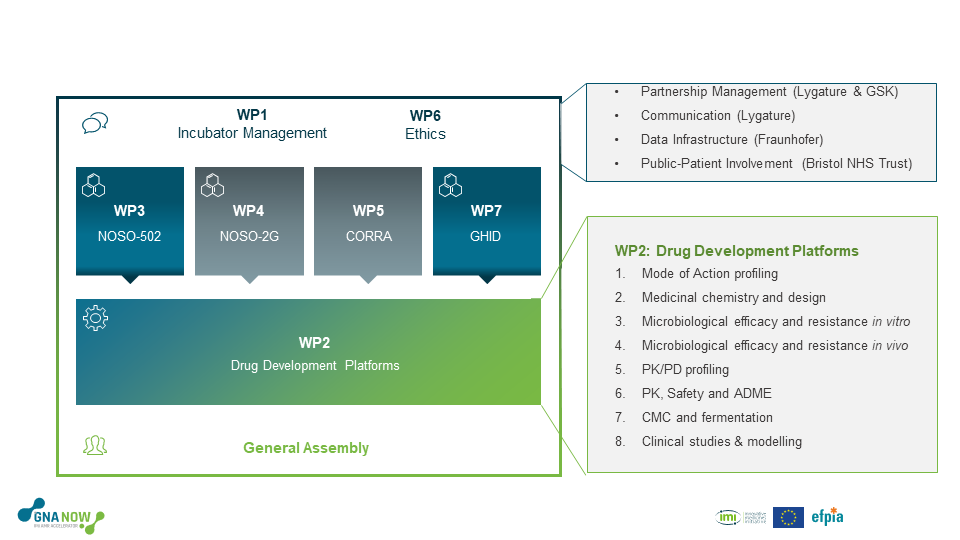
Figure 1: The GNA NOW project started with three drug discovery programmes running in parallel (NOSO-502, NOSO-2G & CORRA), supported by eight drug development platforms. Due to unmet criteria, a no-go decision was issued for the NOSO-2G & CORRA programmes. However, a new programme, Global Health Infectious Disease (GHID), led by new EFPIA partner, GSK, has been included in the project.
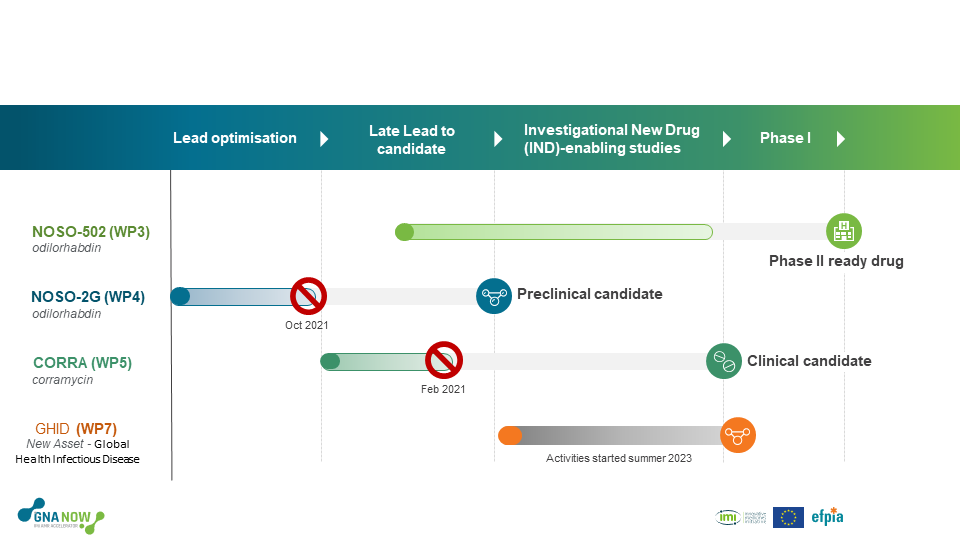
Figure 2: The six-and-a-half-year project has worked on 4 different compounds, with the aim of advancing them further into the clinical development candidate stage. Two compounds (NOSO-2G and CORRA) obtained a no-go status due to unmet scientific criteria.
Governance
The GNA NOW consortium has put in place robust governance and management structures to ensure that the most promising compounds are pursued diligently, to allow for rational assessment of programmes, and to ensure that all stakeholders are heard.
Publications
- Pantel, L., Guérin, F., Serri, M., Gravey, F., Houard, J., Maurent, K., Attwood, M., Noel, A., MacGowan, A., Racine, E., Cattoir, V., Gualtieri, M. (2022) Exploring Cluster-Dependent Antibacterial Activities and Resistance Pathways of NOSO-502 and Colistin against Enterobacter cloacae Complex Species, American Society for Microbiology, Antimicrobial Agents and Chemotherapy, DOI: 10.1128/aac.00776-22
Project consortium
About Lygature
Lygature is a not-for-profit foundation that catalyzes the development of new medical solutions for patients by driving public-private partnerships (PPPs) between academia, industry and society. Lygature has extensive expertise in program management of large pan-European PPPs such as the IMI European Lead Factory (ELF), the IMI RADAR program and the IMI ADAPT SMART program. As independent enabler, Lygature functions as coordinator of GNA NOW and will provide operational, management, financial and legal support to the project. In addition, the development of the consortium’s communication and dissemination strategy is also part of Lygature’s GNA NOW responsibilities, as well as providing a secured web-based IT platform enabling all partners to safely share general project information.
Contact:
Kristina Orrling: kristina.orrling@lygature.org
Lianne Husholf: lianne.hulshof@lygature.org
About GSK
GSK is a science-led global healthcare company that researches and develops a broad range of innovative products. The R&D organization within the company has extensive expertise in designing, executing, analyzing and reporting clinical trials in many therapeutic areas, including infectious diseases. GSK is committed to reporting the results of clinical research that evaluate medicines and vaccines, irrespective of whether the outcomes are perceived to be positive or negative. GSK is an active member of several collaborations focused on developing new treatments for TB, including multi-drug resistant forms of the infection. In 2012, GSK Tres Cantos joined the TB Drug Accelerator Program – a partnership with several pharmaceutical and public-sector research institutions and the Bill & Melinda Gates Foundation, aiming to speed up the discovery of new medicines by collaborating on early stage research.
Contact:
Tim Miles: tim.j.miles@gsk.com
About Nosopharm
Nosopharm is an innovative biotechnology company headquartered in Lyon (France) and specialized in the research and development of new molecules to combat antimicrobial resistance (AMR). The innovative anti-infective drug discovery platform developed by Nosopharm is based on the therapeutic exploitation of a very original bioresource: the bacterial genera Xenorhabdusand Photorhabdus. The mission of Nosopharm is to discover and develop novel first-in-class anti-infectives addressing unmet medical needs.
Contact:
Philippe Villain-Guillot: p.villainguillot@nosopharm.com
Emilie Racine: e.racine@nosopharm.com
About BIOASTER
Created in April 2012 by l’Institut Pasteur and Lyonbiopole health competitiveness cluster, following the initiative of the French government, BIOASTER Technology Research Institute (TRI) is working to develop an unique technological and innovative model to support the latest challenges in microbiology. In particular, BIOASTER aims to fight antimicrobial resistance, improve vaccine safety and efficacy, quickly diagnose infections, and take full advantage of human and animal microbiota.
Contact:
Magali Sarafian: magali.sarafian@bioaster.org
Gilles Courtemanche: Gilles.COURTEMANCHE@bioaster.org
About Helmholtz Centre for Infection Research
The Helmholtz Centre for Infection Research (HZI) and its affiliate Helmholtz Institute for Pharmaceutical Science Saarland (HIPS) will support GNA NOW in several work packages. At HZI, we will apply our mass spectrometry-based technology to quantify compound uptake in subcellular compartments of Gram-negative bacteria. For different antibiotic series under study, the kinetics of uptake in periplasm, cytoplasm and membrane fractions will be determined. These experiments will be conducted in at least four different species of Gram-negative bacteria of high medical interest and defined genetic mutants thereof.
https://www.helmholtz-hzi.de/en/
Contact:
Mark Brönstrup: Mark.Broenstrup@helmholtz-hzi.de
About Helmholtz Institute for Pharmaceutical Research Saarland
The Helmholtz Centre for Infection Research (HZI) and its affiliate Helmholtz Institute for Pharmaceutical Science Saarland (HIPS) will support GNA NOW in several work packages. At HIPS, we aim to establish biotechnological production platforms for antibiotics under study. Emphasis will be given on building blocks difficult to make by total synthesis to achieve a reduction in cost-of-goods. In addition, this work will help generate further derivatives of selected examples. We will employ our in-house platform for target elucidation of natural products by identifying and characterising self-resistance markers of natural producers. In addition, biophysical interaction studies and resistance development in pathogens with subsequent determination of underlying genetic factors will be used.
https://www.helmholtz-hzi.de/en/
Contact:
Rolf Müller: Rolf.Mueller@helmholtz-hips.de
About North Bristol Trust
Within the Department of Infection Sciences at North Bristol NHS Trust (NBT), there is more than 25 years of experience in performing in vitro PK/PD experiments for drug development programmes. NBT will contribute a number of basic microbiology techniques to GNA NOW, such as MIC, MBC, Time Kill Curve experiments, determination of post-antibiotic effects and antibiotic interaction studies. Moreover, patient and public involvement (PPI) is well established in antimicrobial research at NBT. The team are well convinced of the benefits of PPI in both ensuring that research is relevant and appropriate for patients, but also that the quality of research is enhanced by including the patient’s perspective and therefore has an established PPI panel.
Contact:
Alasdair Macgowan: Alasdair.Macgowan@nbt.nhs.uk
Tony Timlin: tony.timlin@nbt.nhs.uk
About University of Liverpool
The Centre for Antimicrobial Pharmacodynamics (CAP) is based in the Department of Clinical and Molecular Pharmacology, Institute of Translational Medicine, University of Liverpool. Antimicrobial resistance is a growing threat and there has been a global push to address to develop new drugs. The CAP is one of relatively few academic laboratories in the world that can develop pharmacodynamic packages for new antimicrobial agents. We study how antimicrobial agents are distributed in the body (pharmacokinetics) and their effect on killing microorganisms (pharmacodynamics). CAP provides preclinical and early phase clinical support to ensure new drugs are developed in a streamlined manner.
Contact:
Shampa Das: Shampa.Das@liverpool.ac.uk
About INSERM
Founded in 1964, Inserm is a public scientific and technological institute which operates under the joint authority of the French Ministries of Health and Research. The institute is dedicated to biomedical research and human health, and is involved in the entire range of activities from the laboratory to the patient’s bedside. It also partners with the most prestigious research institutions in the world that are committed to scientific challenges and progress in these fields.
About Université de Poitiers
The University of Poitiers is symbiotically rooted in its region, getting as close as possible to institutional, economic, cultural and academic actors. The University of Poitiers is located mainly in Greater Poitiers, on the campuses of central Poitiers, Poitiers-East and Futuroscope. It has also developed campuses in Niort and Angoulême, where it offers undergraduate (including the 2-year DUT diplomas) and postgraduate studies. It also has sites in Châtellerault and Segonzac.
Université de Poitiers is involved as a Linked Third Party of INSERM.
Contact:
William Couet: william.couet@univ-poitiers.fr
Sandrine Marchand: Sandrine.Marchand@univ-poitiers.fr
About Erasmus MC
In the different platforms, the Department of Medical Microbiology and Infectious Diseases of the Erasmus MC will focus on determining the pharmacokinetics/dynamics (PK/PD) of the new compounds in different mouse models (e.g. lung infection model, urinary tract infection model, thigh infection model). The drug monitoring of the plasma samples and the subsequent mathematical modeling will be performed in close collaboration with the Department of Pharmacy of the Erasmus MC. Besides determination of PK/PD parameters, Erasmus MC will also perform the in vitro mutation frequency determinations of the compounds for different reference organisms. In addition, an in vitro urinary tract model will be applied to monitor the resistance selection during the course of an infection and subsequent antibiotic treatment.
Contact:
Wil Goessens: w.goessens@erasmusmc.nl
About MedUni Vienna
The Medical University of Vienna (briefly: MedUni Vienna) is one of the most traditional medical training and research facilities in Europe. With its 26 university hospitals, three clinical institutes, 12 theoretical medicine centres and numerous highly specialised laboratories, it is included among the most important cutting-edge research institutes of Europe in the area of biomedicine. The role of MUW in the programme will be to plan, perform and analyse clinical first in man studies in healthy subjects and studies in specific patient populations with all compounds developed by the consortium. The clinical assessment will cover safety and tolerability. After biochemical quantification of the pharmacokinetic samples in a certified laboratory, pharmacokinetic analysis will be performed to optimize preclinical models as well as PK/PD modelling in other work packages.
https://www.meduniwien.ac.at/web/en/
Contact:
Markus Zeitlinger: markus.zeitlinger@meduniwien.ac.at
About Fraunhofer-ITMP
Fraunhofer-ITMP leads the data management aspects of GNA-NOW, supported by the innovative informatics company, GRIT42, which will supply software tools to the consortia. A key part of Fraunhofer’s role is to help increase the impact of GNA-NOW by establishing the FAIR (Findable, Accessible, Interoperable and Reusable) principles in overall data management. These principles underpin how the consortia will work with data generated in each of the platforms. They will help facilitate the use of Machine Learning and similar techniques to extract additional value from the results and support the work of the wider antibiotic drug discovery community.
https://www.itmp.fraunhofer.de
Contact:
Philip Gribbon: Philip.Gribbon@itmp.fraunhofer.de
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853979. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution. https://www.imi.europa.eu/ |