Follow us on:
Mission and Vision
Strategy
The main objective of ERA4TB is to create a European open platform to accelerate the development of new regimens for the treatment of tuberculosis. To reach this goal, the consortium has set the following specific objectives:
- Implementation of state-of-the-art tools and capacities into an open platform for the evaluation of TB drug candidates to effectively progress compounds from early preclinical to clinical development and identify potential new Pan-tuberculosis (Pan-TB)1 regimens ready for Phase II clinical evaluation.
- Development of modelling and simulation tools and application of standard and new artificial intelligence (AI) techniques for better characterization of pharmacokinetic-pharmacodynamic (PK/PD) relationships (concentration-effect or exposure-response relationships depending on the trial), optimization of clinical trial design, prediction of therapeutic dose range and antibacterial activity in humans.
- Management of data generated by the project, integrating also data and knowledge from historical datasets available in reference databases and from previous and existing consortia and projects, in the context of an ever-improving ‘learning system’ that allows to refine the platform continuously.
- Provision of a flexible and efficient management structure able to adapt to the capacity and resource allocation level required by each platform component at each stage of the project, depending on each compound’s progression and attrition dynamics and on inherent variables of the multiple combination assays.
- Provide a sustainability plan that incorporates all the synergies and lessons learned within the project and secures the survival of the platform beyond the life of the project.
- Define and execute an outreach, engagement, dissemination and communication plan in collaboration with regulatory authorities and other stakeholders, including patient organizations, to maximize the impact of the project.
- Hollow fibre systems
- Single-cell time-lapse analysis
- Imaging in animal models
- Novel biomarkers
- Host-pathogen interaction and virulence studies
- Drug-disease modelling
- Physiologically-based predictive modelling
- AI-driven data mining
- Clinical trial simulation techniques
Governance
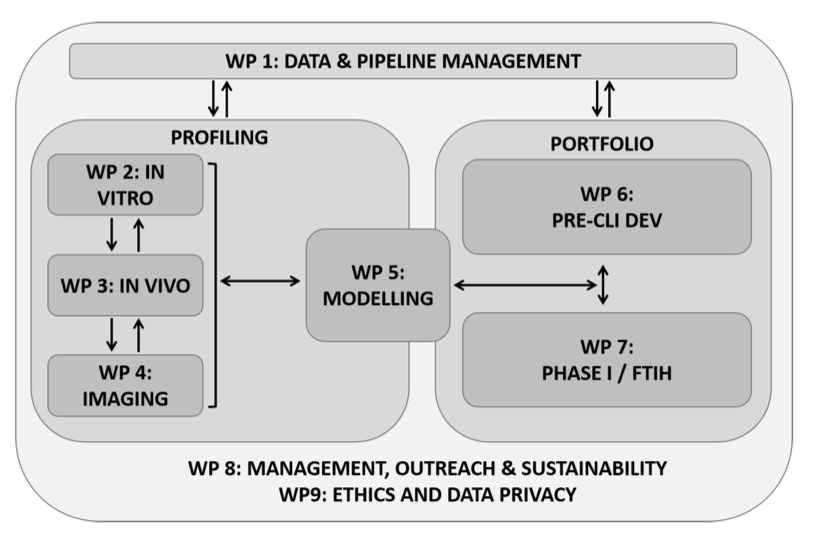
The ERA4TB platform is based on a progression pipeline that can cater for a variety of molecules at different stages of development. The drug candidates entering the pipeline will be supplied by EFPIA and Associated Partners and progress through the required research phases until reaching Phase I FTIH (First Time in Humans) clinical trials.
It is the mission of the consortium to bring to Phase I the maximum number of molecules suitable for inclusion in new anti-TB regimens. In view of this, the project’s development activities have been divided into two main integrated areas: Preclinical Profilingand Portfolio Development, the latest including pharma Development and Phase I FTIH. The separation of these activities allows the Consortium to develop a comprehensive work plan that accommodates the different entry points for the various molecules, ensuring flexibility to identify the expertise required and adjust resource allocation and workload distribution appropriately.
Publications
- Karakitsios E, della Pasqua O, Dokoumetzidis A. Extrapolation of lung pharmacokinetics of bedaquiline across species using physiologically-based pharmacokinetic modelling. British Journal of Clinical Pharmacology. 2025;91(11). doi:10.1002/BCP.70163
- Rabodoarivelo MS, Hoffmann E, Gaudin C, et al. Protocol to quantify bacterial burden in time-kill assays using colony-forming units and most probable number readouts for Mycobacterium tuberculosis. STAR Protocols. 2025;6(1):103643. doi:10.1016/j.xpro.2025.103643
- Fernow J, Olliver M, Couet W, et al. The AMR Accelerator: from individual organizations to efficient antibiotic development partnerships. Nature Reviews Drug Discovery 2024. Published online September 23, 2024. doi:10.1038/d41573-024-00138-9. Green Open Access available through DiVA.
-
Gries R, Chhen J, van Gumpel E, et al. Discovery of dual-active ethionamide boosters inhibiting the Mycobacterium tuberculosis ESX-1 secretion system. Cell Chemical Biology. 2024;31(4):699-711.e6. doi:10.1016/j.chembiol.2023.12.007
- Nagar S, Nicholls D, Dawoud D, et al. A systematic review of economic evaluations of pharmacological treatments for active tuberculosis. Frontiers in Public Health. 2024;12:1201512. doi:10.3389/FPUBH.2024.1201512
-
Aguilar-Ayala DA, Fernando Sanz-García |, Marie |, et al. Evaluation of critical parameters in the hollow-fibre system for tuberculosis: A case study of moxifloxacin. British Journal of Clinical Pharmacology. Published online April 17, 2024. doi:10.1111/BCP.16068
-
Moraga P, Prieto P, Conradie A, et al. Academia and industry agreement on a feasibility tool for first-time-in-human clinical trial units. Clinical and Translational Science. 2023;16(12):2421-2428. doi:10.1111/CTS.13655
-
van Wijk RC, Lucía A, Sudhakar PK, et al. Implementing best practices on data generation and reporting of Mycobacterium tuberculosis in vitro assays within the ERA4TB consortium. iScience. 2023;26(4). doi:10.1016/j.isci.2023.106411
-
Toniolo C, Dhar N, McKinney JD. Uptake‐independent killing of macrophages by extracellular Mycobacterium tuberculosis aggregates. The EMBO Journal. 2023;42(9). doi:10.15252/embj.2023113490
-
Mishra R, Hannebelle M, Patil VP, et al. Mechanopathology of biofilm-like Mycobacterium tuberculosis cords. Cell. 2023;186(23):5135-5150.e28. doi:10.1016/j.cell.2023.09.016
-
Gries R, Dal Molin M, Chhen J, et al. Characterization of Two Novel Inhibitors of the Mycobacterium tuberculosis Cytochrome bc 1 Complex. Antimicrobial Agents and Chemotherapy. 2023;67(7). doi:10.1128/aac.00251-23
- Mistretta M, Gangneux N, Manina G. Microfluidic dose–response platform to track the dynamics of drug response in single mycobacterial cells. Scientific Reports 2022 12:1. 2022;12(1):1-18. doi:10.1038/s41598-022-24175-9
- Visuña L, Yang D, Garcia-Blas J, Carretero J. Computer-aided diagnostic for classifying chest X-ray images using deep ensemble learning. BMC Medical Imaging. 2022;22(1):178. doi:10.1186/s12880-022-00904-4
- Griego A, Douché T, Gianetto QG, Matondo M, Manina G. RNase E and HupB dynamics foster mycobacterial cell homeostasis and fitness. iScience. 2022;25(5):104233. doi:10.1016/j.isci.2022.104233
-
Thacker V v., Dhar N, Sharma K, Barrile R, Karalis K, McKinney JD. A lung-on-chip model of early M. tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLife. 2020;9:1-73. doi:10.7554/ELIFE.59961
-
Faraj A, Clewe O, Svensson RJ, Mukamolova G v., Barer MR, Simonsson USH. Difference in Persistent Tuberculosis Bacteria between In Vitro and Sputum from Patients: Implications for Translational Predictions. Scientific Reports 2020 10:1. 2020;10(1):1-10. doi:10.1038/s41598-020-72472-y
Project consortium
Partners
The ERA4TB Consortium brings together a multi-disciplinary team with proven expertise and capabilities in TB drug development to profile and progress anti-TB compounds up to completion of Phase I.
The project consortium integrates 31 organizations, namely eight prestigious academic institutions (UC3M, UNIZAR, UU, EPFL, UKÖ, UNIPD, UPV, LUND), four non-profit organizations (IPP, IPL, iM4TB, BAR), eight public research organizations (FZB, CNR, IDMIT, SERMAS, PHE, NICE, SCI, IOS) and five highly skilled small-medium enterprises (SYNAPSE, C-Path, IBT, QPS, GRIT), together with three EFPIA members (GSK, EVT, JANSSEN), and three IMI2 Associated Partners (BMGF, TBA, DDU).
| Logo | Short name | Partners full name | Link |
|---|---|---|---|
 |
UC3M | University Carlos III Madrid | https://www.uc3m.es/Home |
 |
IPP | Institute Pasteur (Paris) | https://www.pasteur.fr/en/institut-pasteur |
 |
UNIZAR | University of Zaragoza / ARAID | www.unizar.es |
 |
IPL | Institute Pasteur de Lille Foundation | https://www.pasteur-lille.fr/5/research/units/ |
 |
SYNAPSE | Synapse Managers Partners | https://synapse-managers.com/ |
 |
BOR | Forschungszentrum Borstel | https://fz-borstel.de/index.php/de/ |
 |
IM4TB | Foundation Innovative Medicines for Tuberculosis | http://im4tb.org |
 |
C-Path | Critical Path Institute, Limited | https://c-path.eu/ |
 |
CNR | Consiglio Nazionale delle Richerche | https://www.iac.cnr.it/ |
 |
IDMIT | Infectious Diseases Models for Innovative Therapies | https://idmitcenter.fr/symposium/ |
 |
SERMAS | Instituto de Investigación Hospital Universitario La Paz | http://www.idipaz.es |
 |
UU | Uppsala University | https://www.uu.se/en |
 |
PHE | Public Health England- Department of Health | https://www.gov.uk/government/organisations/public-health-england/about |
 |
EPFL | École polytechnique fédérale de Lausanne | https://www.epfl.ch/labs/mckinney-lab/ |
 |
UKÖ | University of Köln | http://www.uni-koeln.de |
 |
UNIPD | University of Padova | https://www.unipd.it/en/ |
 |
UPV | University of Pavia | https://web.unipv.it |
 |
NICE | The National Institute for Health and Care Excellence | https://www.nice.org.uk/about |
 |
IBT | ImaBiotech | www.imabiotech.com |
 |
SCI | Sciensano | https://www.sciensano.be/en/about-sciensano/sciensanos-organogram/bacterial-diseases/tuberculosis-and-mycobacteria#want-to-know-more- |
 |
LIOS | Latvia Institute of Organic Synthesis | http://www.osi.lv/en/. |
 |
BAR | Bioaster Foundation de Coopération Scientifique | https://www.bioaster.org/bioaster/bioaster-community/ |
 |
QPS | QPS Netherlands BV | https://www.qps.com |
 |
LUND | Phase I Unit of Skane Hospital | https://sodrasjukvardsregionen.se/clinicaltrialunit/ |
 |
GSK | Glaxosmithkline Investigacion y Desarrollo SL | https://www.gsk.com/ |
 |
EVOTEC | Evotec International GmbH | https://www.evotec.com/en |
 |
Janssen | Janssen Pharmaceutica NV | https://www.janssen.com/ |
 |
BMGF | Bill & Melinda Gates Foundation | https://www.gatesfoundation.org/ |
 |
TBA | Global Alliance for TB Drug Development | https://www.tballiance.org/ |
 |
DDU | University of Dundee | http://www.drugdiscovery.dundee.ac.uk/ |
 |
GRIT42 | Grit systems | https://grit42.com |
 |
CIM – Sant Pau | IIB Sant Pau | http://www.recercasantpau.cat/en/ |
ERA4TB Project Technical Coordinator
Dr. Juan Jose Vaquero
University Carlos III de Madrid
Calle Madrid 126
28 903 Madrid, Spain
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 853989. The JU receives support from the European Union’s Horizon 2020 research and innovation programme, and in-kind support from EFPIA, Global Alliance for TB Drug Development, Bill & Melinda Gates Foundation and University of Dundee. https://www.imi.europa.eu/ |