The AMR Accelerator calls to action: European capacity for antibiotic R&D requires long-term funding
Antibiotic resistance is a global public health threat. Modern health systems rely on antibiotics to prevent and treat infections, and the need for new drugs is urgent. In a joint call to action, the AMR Accelerator projects ask for long-term investments, emphasising the need to preserve the European capacity for antibiotic R&D by sustaining the assets, expertise, and research infrastructures required to develop new treatments for drug-resistant infections.
The need for new antibiotics is well-recognized, and on 26 September, the United Nations General Assembly will accelerate political actions in a high-level meeting on antimicrobial resistance, or ‘AMR’. The AMR Accelerator – a public-private partnership involving nine European projects and 98 organisations – urges government leaders and private actors to invest in the development of antibiotics and research on antimicrobial resistance.
The call to action, published in Nature Reviews Drug Discovery, emphasises the need for coordinated action and commitments to meet the threat of antimicrobial resistance and secure a sustainable future for European antibiotic development. The return on investment is low, and many large pharmaceutical companies have abandoned the field. According to the authors, collaboration and risk-sharing can help keep companies in anti-infective drug development.
“Without a long-term funding strategy for antibiotics research and development, there is a significant risk that the AMR Accelerator’s efforts to progress the antibacterial pipeline are lost,” says Anders Karlén, Professor of Computer-Aided Drug Design at Uppsala University and Coordinator of the COMBINE project, responsible for bringing the AMR Accelerator projects together.
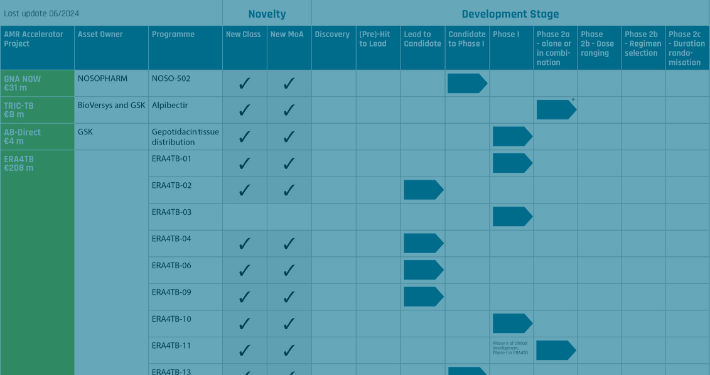
The AMR Accelerator portfolio ranges from discovery to phase II clinical trials, and covers both tuberculosis and Gram-negative bacteria. It represents a substantial investment from the European Commission and pharmaceutical industry. Funded by the Innovative Medicines Initiative, the AMR Accelerator projects have a combined budget of €479 million. Together, they have progressed 44 antibacterial programmes over the past 5 years, and to date, the efforts have resulted in two completed Phase I studies, and five ongoing Phase I and II studies. However, the funding is only temporary.
“The AMR Accelerator has successfully accelerated the development of promising therapeutics, but we need continued commitment to ensure that the antibiotics reach the next stage of development and, ultimately, the patients who need them”, says Anders Karlén.
The success of the AMR Accelerator demonstrates the value of public-private partnerships by strengthening the antibiotic pipeline and providing tools and infrastructure for the global AMR research community. The AMR Accelerator has built a critical mass and created synergies that enable organisations to share expertise and resources. The results are tangible: high-quality science, a stronger antibiotic pipeline, and a legacy of research infrastructures, including standardised infection models, clinical trial networks, and open data resources. The key challenge for all nine projects is to ensure the long-term sustainability of assets, infrastructures, and expertise. The AMR Accelerator delivers a clear message: we need commitment and investment from governments, industry, and other stakeholders to protect our capacity to develop life-saving antibiotics.
The call to action was published in the comment section of Nature Reviews Drug Discovery. Green Open Access available through DiVA.
The AMR Accelerator: from individual organizations to efficient antibiotics development partnerships: https://www.nature.com/articles/d41573-024-00138-9.
For questions, please contact
Anders Karlén, Professor of Computer-Aided Drug Design, Uppsala University and COMBINE Coordinator.
Email: anders.karlen@ilk.uu.se, Phone: +46 18 471 42 93 / +46 70-167 91 77
Marie Olliver, COMBINE Alliance Manager, Uppsala University
Email: marie.olliver@ilk.uu.se
About the AMR Accelerator
The AMR Accelerator programme was launched in 2019, with the aim to accelerate the development of medicines for patients suffering from infections with drug-resistant Mycobacterium tuberculosis, nontuberculous mycobacteria (NTM), and Gram-negative bacteria, and build capability for antibiotics research and development. The programme is funded by the Innovative Medicines Initiative (IMI). The AMR Accelerator programme includes nine projects: AB-Direct, COMBINE, ERA4TB, GNA NOW, PrIMAVeRa, RespiriNTM, RespiriTB, TRIC-TB, and UNITE4TB. Together, the projects have a €479 million budget. The 98 partners represent key stakeholders from academia, industry, small- and medium-sized companies, patient organisations, regulators, and Health Technology Assessment.
About COMBINE
COMBINE has a coordinating role in the AMR Accelerator, and a scientific mission aiming to improve 1) the design and analysis of clinical trials, and 2) animal infection model reproducibility and translation to clinical efficacy. COMBINE has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853967.
About GNA NOW
Established in 2019, the GNA NOW Consortium (Gram-Negative Antibacterials NOW), is a joint initiative of 11 partners from industry and academia, aimed at delivering Gram-negative antibacterials with new modes of action to the clinical phase. The Consortium is focused on progressing treatments for severe diarrhoea, targeting four enteric pathogens: Shigella, Campylobacter, E. coli, and Salmonella. The Consortium consists of GSK (Scientific Lead), BioAster, Erasmus MC, Fraunhofer, Helmholtz Centre for Infection Research, Inserm, Medical University of Vienna, North Bristol NHS Trust, University of Liverpool, University of Poitiers, and Lygature (Coordinator).
Find out more: www.amr-accelerator.eu/project/gna-now/
About ERA4TB
The ERA4TB (European Accelerator of Tuberculosis Regime) project is devoted to creating a European Open platform to accelerate the development of new treatment regimens for tuberculosis. ERA4TB is expected to revolutionize the way in which tuberculosis treatments are developed thanks to its parallelized, multi-entry pipeline structure. By the end of the project the consortium expects to have developed at least two or more new combination regimens with treatment-shortening potential ready for Phase II clinical evaluation.
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 853989. The JU receives support from the European Union’s Horizon 2020 research and innovation programme, EFPIA, the Global Alliance for TB Drug Development non-profit organisation, the Bill & Melinda Gates Foundation, and the University of Dundee.
Juan Jose Vaquero, Professor of Bioengineering and ERA4TB Technical Coordinator, Universidad Carlos III de Madrid.
Email: juanjose.vaquero@uc3m.es
Katharine Cresswell, ERA4TB communications and engagement, National Institute for Health and Care Excellence.
Email: Katharine.cresswell@nice.org.uk
About PrIMAVeRa
PrIMAVeRa (Predicting the Impact of Monoclonal Antibodies & Vaccines on Antimicrobial Resistance) is dedicated to creating advanced mathematical models that predict how vaccines and monoclonal antibodies (mAbs) can reduce antimicrobial resistance (AMR). By providing a clearer understanding of the most effective strategies for vaccine and mAb implementation, PrIMAVeRa aims to empower public health officials and policymakers to make informed decisions that maximize public health benefits and economic gains.
PrIMAVeRa has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 101034420. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
https://www.primavera-amr.eu/
Email: communication@euvaccine.eu
About RespiriNTM & RespiriTB
Launched on the 1st of May 2019 the RespiriTB & RespiriNTM consortium are on an 8-year journey exploring multiple approaches to determine new targets for anti-mycobacterial compounds, define and optimize novel inhibitors and move these through the process of hit-to-lead compound up until the First-in-Human trials. The research teams that make up the RespiriTB & RespiriNTM are working closely together to identify new solutions in the fight against the deadly bacteria Mycobacterium tuberculosis and the related bacterial species M. avium, and M. abscessus.
Meindert Lamers, Project Coordinator, RespiriTB & RespiriNTM, Leiden University Medical Centre
Email: m.h.lamers@lumc.nl
Illina Bareja, Communication Manager, RespiriTB & RespiriNTM, FFUND BV.
Email: ilina.bareja@ffund.nl
About TRIC-TB
TRIC-TB successfully completed the Phase 1 study with alpibectir (BVL-GSK098), a small molecule potentiating the activity of ethionamide. Alpibectir allows to lower the efficacious human dose of ethionamide minimizing dose-dependent side effects and improve patient compliance. The combination alpibectir/ethionamide (AlpE) is rapidly bactericidal, overcomes MDR and Eto-resistance, and has the potential to become an important building block in novel regimens. AlpE is currently tested in Phase 2a proof-of-concept study (NCT05473195).
TRIC-TB has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853800. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in-kind contribution.
Michel Pieren, Clinical Program Team Leader, BioVersys AG & project coordinator, TRIC-TB.
E-mail: michel.pieren@bioversys.com
About UNITE4TB
UNITE4TB is one of the nine projects within the AMR Accelerator. Working across a global clinical trials network, UNITE4TB conducts regulatory standard Phase 2 clinical trials to accelerate clinical evaluation of novel drugs and combinations of drugs for tuberculosis (TB). Innovative adaptive trial designs, treatment response biomarkers, pharmacokinetic-pharmacodynamic models and Artificial Intelligence / Deep Learning techniques are integrated across study protocols, deploying cutting-edge methodologies to find antibiotic regimens with the highest likelihood of improved clinical efficacy.
UNITE4TB has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 101007873. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA, Deutsches Zentrum für Infektionsforschung e. V. (DZIF), and Ludwig-Maximilians-Universität München (LMU). EFPIA/AP contribute to 50% of funding, whereas the contribution of DZIF and the LMU University Hospital Munich has been granted by the German Federal Ministry of Education and Research.
www.unite4tb.org
Email: communications@unite4tb.org