Mission and Vision
The ultimate goal of PrIMAVeRa (Predicting the Impact of Monoclonal Antibodies & Vaccines on Antimicrobial Resistance) is to develop comprehensive mathematical models to predict the impact of vaccines and monoclonal antibodies (mAbs) on the reduction of AMR, thereby enabling decision-makers to prioritise the most promising new vaccines and mAbs. A sustainability plan will also be designed by the project partners to ensure long-term access to the project results, including models, beyond the duration of the PrIMAVeRa project.
PrIMAVeRa’s mathematical models and epidemiological repository will help illuminate areas previously obscured due to lack of information and provide an indication of which strategies (or combinations thereof) of vaccine and mAb implementation are most likely to result in the greatest benefit to public health. In short, PrIMAVeRa will enable public health officials and policy makers to reach the decisions resulting in the highest net benefit to the public health and economies of their respective areas. This would increase investment to vaccines and mAbs development. Additionally, overall, it would help preserving last resort antimicrobials.
Strategy
PrIMAVeRA seeks to develop mathematical models and an epidemiological repository to assess the impact of vaccines and monoclonal antibodies (mAbs) on antimicrobial resistance (AMR) – one of the top ten threats to global health as determined by the World Health Organization (WHO).
This five-year project (01 November 2021 – 31 October 2026) brings together global leaders in the fields of computational and mathematical modelling, epidemiology, statistics, public health and health economics, microbiology, database management, data science, vaccine and monoclonal antibody research.
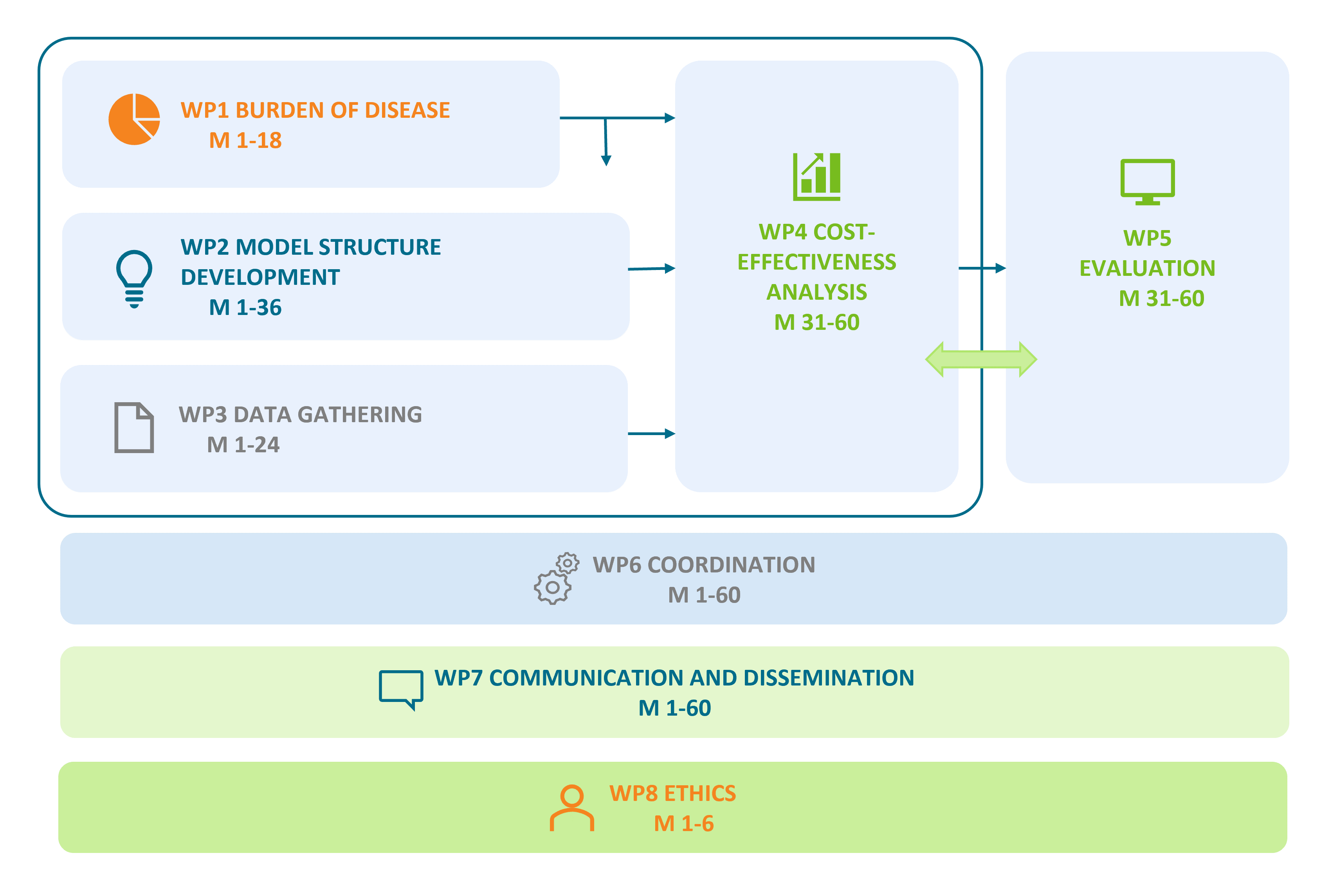
This project is structured in 8 work packages (WP):
- WP1: Burden of Disease
- WP2: Model Structure Development
- WP3: Data Gathering
- WP4: Cost-Effectiveness Analyses
- WP5: Evaluation
- WP6: Coordination
- WP7: Communication & Dissemination
- WP8: Ethics
Governance
The organisational structure will include three types of consortium bodies: (1) Strategic Bodies, (2) Implementation Bodies, (3) an Advisory Body.
Publications
- Diletta Pezzani, M., Arieti, F., Babu Rajendran, N., Barana, B., Cappelli, E., De Rui, M. E., Galia, L., Hassoun-Kheir, N., Argante, L., Schmidt, J., Rodriguez-Bano, J., Harbarth, S., de Kraker, M., Primrose Gladstone, B., Tacconelli, E. (2023) Frequency of bloodstream infections caused by six key antibiotic-resistant pathogens for prioritization of research and discovery of new therapies in Europe: a systematic review, ScienceDirect, European Society of Clinical Microbiology and Infectious Diseases, DOI: 10.1016/j.cmi.2023.10.019 /FULL ARTICLE ONLY ACCESSIBLE TO ESCMID MEMBERS AND SUBSCRIBERS/
- Robotham, J. V., Tacconelli, E., Vella, V., de Kraker, M. E. A. (2023), Synthesizing pathogen- and infection-specific estimates of the burden of antimicrobial resistance in Europe for health-technology assessment: gaps, heterogeneity, and bias, ScienceDirect, European Society of Clinical Microbiology and Infectious Diseases, DOI: 10.1016/j.cmi.2023.10.004 /FULL ARTICLE ONLY ACCESSIBLE TO ESCMID MEMBERS AND SUBSCRIBERS/
- Hassoun-Kheir, N., Guedes, M., Ngo Nsoga, M.-T., Arguante, L., Arieti, F., Gladstone, B. P., Kingston, R., Naylor, N. R., Pezzani, M. D., Pouwels, K. B., Robotham, J. V., Rodríguez-Baño, J., Tacconelli, E., Vella, V., Harbarth, S., de Kraker, M. E. A. (2023) A systematic review on the excess health risk of antibiotic-resistant bloodstream infections for six key pathogens in Europe, ScienceDirect, European Society of Clinical Microbiology and Infectious Diseases, DOI: 10.1016/j.cmi.2023.09.001
- Hassoun-Kheir, N., Buetti, N., Olivier, V., Perez, M., Frossard, J., Renzi, G., Schrenzel, J., Saudan, P., Harbarth, S. (2023) Targeted mupirocin-based decolonization for Staphylococcus aureus carriers and the subsequent risk of mupirocin resistance in haemodialysis patients – a longitudinal study over 20 years, ScienceDirect, Journal of Hospital Infection, DOI: 10.1016/j.jhin.2023.01.019
- Hassoun-Kheir, N., Harbarth, S. (2022) Estimating antimicrobial resistance burden in Europe—what are the next steps?, The Lancet, Public Health, DOI: 10.1016/S2468-2667(22)00250-X
Project consortium
About European Vaccine Initiative
EVI is a non-profit organisation with a mission to accelerate the development of vaccines for global health. EVI’s expertise includes preclinical/ clinical vaccine development and coordination of large-scale collaborative projects funded by EC, IMI, EDCTP and other funders.
Link to website:
www.euvaccine.eu
About Universitair Medisch Centrum Utrecht
The UMC Utrecht is an internationally renowned research and teaching hospital with 1,200 beds and all medical specialties represented. Within the Department of Medical Microbiology and the “Julius Centre for Health Sciences and Primary Care” more than 50 researchers from different medical specialties collaborate in studying antibiotic resistance, epidemiology of infectious diseases and ICU-acquired infections, applying molecular, microbiological, epidemiological and theoretical methods. Both departments harbour extensive experience with large-scale multi-centre intervention trials and epidemiological analyses of large patient databases. UMCU is the Coordinator of 4 COMBACTE projects and leads the WP on the hospital-based clinical study in ECRAID-Base, and VALUE-Dx. Within COMBACTE-NET, and led by UMCU, a high-quality clinical research network for new options to treat or prevent bacterial infections in European Union member states and affiliated countries was created, which was named CLIN-Net. Within this network 937 clinicians across Europe have been GCP-trained, a certification of clinical study sites has been established, and an extensive up-to-date database of research facilities, research interests and past performance of its member sites in CLIN-net trials has been maintained. CLIN-Net has optimized a rapid feasibility assessment and site selection process for clinical studies and has developed (and maintained) excellent communication and dissemination of COMBACTE activities within and outside the consortium. As of now the CLIN-Net holds a database of 3.286 contacts in 1010 hospitals in 42 countries. Of these hospitals, 727 have participated in feasibility assessment and site selection process and 354 have enrolled patients in any of the 23 studies in which CLIN-Net has been involved, and in which 24,879 patients have been enrolled.
Link to website:
www.umcutrecht.nl
About Université de Genève
The Hopitaux Universitaires de Genève (HUG) is the UNIGE-affiliated university hospital. HUG’s work and research personnel is closely linked to UNIGE’s Faculty of Medicine, and it is recognized nationally and internationally in many cutting-edge disciplines. Several experts (Drs Pittet, Harbarth, and Zingg) have generated ground-breaking articles in the fields of infection control, infection control practices, control of antimicrobial resistance and hand hygiene in particular. The infection prevention and control programme at HUG is the WHO Collaborating Center on Patient Safety and Antimicrobial Surveillance and has coordinated the development of a protocol for a harmonized, global approach to AMR burden estimates. HUG also has a well-set Clinical Research Center whose mission is to promote clinical research within the HUG and the Faculty of Medicine by developing translational research in line with the priority axes defined by the two institutions (HUG and Faculty of Medicine of the University of Geneva). The centre is organized into three units: a Methodological Support unit, a Clinical Investigation Unit, and a Quality Unit.
Link to website:
www.unige.ch
About Università di Verona
The University of Verona is a public university focusing on innovation and research is an internationally renowned research center in the area of biomedical and infectious diseases research. The University Hospital is 1,600-bed medical center including all medical specialties. The Infectious Diseases Section, within the Dept. of Diagnostics and Public Health, has extensive clinical and research activities with a multidisciplinary team involving more than 80 members with different specialities (infectious diseases, internal medicine, microbiology, biology, statistics, bio-statistics, infection control, psychology) coordinating and participating in several national and international projects funded by a range of different donor programmes (e.g. WHO, GARDP, IMI, Horizon2020, JPI-AMR, AIFA, and ESCMID). Research topics include AMR transmission and Burden, Antibiotic Stewardship, Diagnostic Stewardship and Public Health. The research group contributed to the development of the TREAT software (funded through EU FP7) one of the first examples of application of intelligence technology to provide recommendations on antibiotic therapy joining evidence data and calibrated local level. The research team also led one of the biggest multi-center cohort studies to define the impact of antibiotics on intestinal flora and acquisition of antibiotic resistant bacteria, applying machine learning for the first time in assessing patient risk by antibiotic. The group is highly experienced in leading guidance document and systematic reviews and meta-analyses. One of the most recent reviews confirmed the impact of stewardship on resistant infections (Lancet Infect Dis 2018). The document has been included in major public health documents to implement stewardship interventions. The team leads EPI-NET (the epidemiological pillar of COMBACTEMAGNET) and the JPI-AMR ARCH Net (One Health Approach) and the qualitative systematic review of studies assessing the AMR burden for WHO/GLASS.
Link to website:
www.univr.it
About Danmarks Tekniske Universitet
DTU is the largest technical university in Denmark with more than 5,000 employees organized in 19 institutes and 9 centers. DTU-Food employs 420 people working in six departments and focuses on public health in relation to human nutrition, food safety and environment. The Institute is part of the national food safety contingency plan and national reference laboratory for microbial food safety. DTU-Food also serves as an international reference laboratory for the EU, WHO (World Health Organisation) and EFSA (European Food Safety Authority) in a number of areas. The Genomic Epidemiology Unit within DTU-Food, is a reference laboratory for EU and FAO on AMR, and WHO on AMR and Genomics. DTU (Frank Aarestrup) is the co-Coordinator of VEO and a partner in ECRAID-Plan.
Link to website:
www.dtu.dk
About Institut Pasteur
The Institut Pasteur is a private, state-approved non-profit foundation for biomedical research which was established in 1887 by Louis Pasteur and hosts some 2,782 people including approximately 600 tenured scientists, several hundred PhD students and post-doctoral fellows, dedicated engineers and technicians, as well as administrative staff. Its mission is built upon three cornerstones: research, teaching and public health. The Institut Pasteur’s public health mission, coordinated inside the Global Health department, is to promote the transfer of scientific discoveries made in its research laboratories to human health applications. It is actively involved in clinical research projects aimed at developing innovative therapies. As a microbiological observatory for communicable diseases, furthermore, it hosts 14 National Reference Centers in Paris (plus 4 with French Guyana), 6 WHO Collaborating Centers, 1 Laboratory for Urgent Response to Biological Threats and 1 Biological Resource Centre. The Epidemiology and Modelling of Antibacterial Evasion (EMAE) unit belongs to the Department of Global Health of the Institut Pasteur. Research is based on investigations we pilot in hospital settings and in communities within low-income countries, and on French population-wide health databases and health data warehouses. Beyond the traditional investigations and analysis techniques (biostatistics and biomathematics), we develop more innovating methodological approaches initiated within our research group in recent years as a result of (1) dynamic contact monitoring, (2) space– time analyses and micro–spatial simulation, (3) dynamic network analysis and agent-based simulation.
Link to website:
www.pasteur.fr
About Servicio Andaluz de Salud
The Andalusian Health Service (Servicio Andaluz de Salud, SAS) is a public body providing all healthcare services to the 8.4 million inhabitants in Andalusia. It is an autonomous “arms-length” organisation, attached to the Ministry of Health of the Andalusian regional Government. The SAS Hospital participating in the project is Hospital Universitario Virgen Macarena (HUVM), a 900-bed reference hospital located in Sevilla. It hosts the coordination of the Andalusian Network for Clinical Research in Infectious Diseases (ANCRAID) and the Spanish Network for Research in Infectious Diseases (REIPI), funded by the regional and national governments, respectively. The Joint Department of Infectious Diseases and Clinical Microbiology is internationally recognized with a vast experience in the field of antimicrobial resistance, antimicrobial use and infection control; it includes a multidisciplinary research group formed by basic scientists, clinical microbiologists, pharmacists and infectious diseases researchers performing basic and clinical research in this field. The research group is part of the Institute of Biomedicine of Seville (IBiS), a multidisciplinary biomedical research centre focused on the development of basic, clinical and translational biomedical research. Research at HUVM is managed by the Fundación Pública Andaluza para la Gestión de la Investigación en Salud de Sevilla (FISEVI; http://fisevi.com/), a non-profit foundation that manages, supports, and develops part of the research and innovation activities carried out in the Andalusian Health Service in Seville according to the Collaboration Agreement between the Andalusian Health Service and the Research Managing Foundations of the Andalusian Public Health System signed in 2012.
Link to website:
www.sspa.juntadeandalucia.es/servicioandaluzdesalud
About Kauno Klinikos
The hospital of the Lithuanian University of Health Sciences (LSMU) Kauno Klinikos is the largest healthcare institution in Lithuania with 2300 beds, covering different fields of medicine. The hospital has 39 departments devoted to all medical and surgical specialties, including cardiovascular surgery, neurosurgery, and organ transplant surgery. The Laboratory Medicine Department and Infection Control Service take leadership in informing the politics of antimicrobial usage, multidrug resistant microorganisms’ control and infection prevention.
Link to website:
https://kaunoklinikos.lt
About UK Health Security Agency
UK Health Security Agency – formerly Public Health England – an Executive Agency of the Department of Health and Social Care, short name UKHSA (previously PHE) is the public health institute of England and exists to protect and improve the nation’s health and wellbeing, and reduce health inequalities, through world-leading science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. The project would crosscut two Divisions of the National Infection Service of UKHSA: Data and Analytical Sciences and HCAI & AMR. The Modelling and Economics Unit within the Data and Analytical Sciences Division comprises infectious disease modellers and health economists with international reputations in the specialist field of modelling infectious diseases. They are working on diverse projects relating to the burden of infectious disease and modelling the impact of interventions against infections in terms of effectiveness and cost-effectiveness, and work both nationally and internationally. The Modelling and Economics Unit is a world leader in its field and has numerous long-standing collaborative links with external academic and non-academic institutes worldwide. It has a large portfolio of policy and public health decision-making its modelling has informed, including vaccination and AMR strategies. The mission of the HCAI & AMR Division in the National Infection Service is to protect people from healthcare-associated and antimicrobial resistant infections, through world-leading public health microbiology, outbreak response, surveillance, research and interventions.
Link to website:
www.gov.uk/government/organisations/uk-health-security-agency
About Instituto de Salud Carlos III
The ISCIII is the main Public Research Entity funding, managing and carrying out biomedical research in Spain, responsible for managing Spain’s Health Research and Development Strategy within the framework of the National R+D+I Plan. The National Microbiology Centre (CNM), is dedicated to research on prevention, diagnosis and treatment of human infectious diseases and additionally, it acts as support for the National Health System by offering services on diagnosis and reference related to human infectious pathogens.
Link to website:
www.isciii.es/Paginas/Inicio.aspx
About Ares Genetics, GmbH
ARES is a Vienna-based digital diagnostics start-up working towards revolutionizing infectious disease diagnostics and therapeutics by artificial intelligence-based DNA testing.
Link to website:
www.opgen.com/ares
About Université Grenoble Alpes
In previous years, the team of Prof. Dr. Pascal Poignard has developed a strong expertise in the isolation of human monoclonal antibodies, being notably among the first to isolate broad-spectrum antibodies neutralizing HIV-1. These antibodies are now being tested in humans and represent a new hope for therapy. Moreover, they represent crucial tools for vaccine development. Combined approaches of reverse vaccinology and structural biology have in particular enabled the isolation and optimization of these antibodies. Current ongoing projects in collaboration with Dr Caspar, a specialist in bacterial resistance at the Grenoble Alpes University Hospital, aim to use the expertise gained in this field to produce therapeutic human monoclonal antibodies against multi-resistant bacteria, especially multi-resistant Gram-negative bacteria. The team has access to the collection of biological samples from the laboratory of bacteriology of Grenoble Alpes University Hospital.
Link to website:
www.univ-grenoble-alpes.fr
About University of Oxford
The University of Oxford is a leading academic institution, at the forefront of scientific research, and consistently rated amongst the world’s best universities. In the recent UK research assessment exercise, UOXF was ranked top in the UK for overall quality of submissions in Clinical Medicine. UOXF is represented by The Centre for Tropical Medicine and Global Health (CTMGH) and the Health Economics Research Centre (HERC). CTMGH hosts over 20 researchers with expertise in mathematical modelling, and antimicrobial resistance is a key research theme. HERC has been at the forefront in health economics since its creation in 1997. HERC harbours extensive experience with the design and economic evaluation of large-scale multi-centre intervention trials and model-based evaluation, preference elicitation through discrete choice experiments and analyses of large patient databases.
Link to website:
www.ox.ac.uk
About Life Science Network
Life Science Network gGmbH (LSN) is a non-profit organisation founded in 2011 in Heidelberg, Germany. The mission of LSN is to support the scientific community and stimulate scientific progress by providing scientists with innovative web-based tools and solutions. To date, LSN developed ten platforms dealing with networking, knowledge sharing, data visualization and communication. Over 70.000 researchers registered an account, and millions of others visited the web platforms developed by LSN since their launch.
Link to website:
www.lifescience.net
About Eberhard Karls Universität Tübingen
The Eberhard Karls University is one of the oldest universities in Europe marked by its success in the German federal and state governments’ Excellence Initiative. The 1585-bedded University Hospital Tübingen was founded in 1805, and is one of the leading centres in German health research. The infectious diseases research group within the University Hospital Tübingen is highly experienced in conducting clinical research, epidemiological studies, and data management. The research group has a multidisciplinary team including infectious disease experts, biologists, epidemiologists coordinating and participating in several national and international projects focussed on healthcare associated infections, antimicrobial resistance and antimicrobial stewardship.
Link to website:
https://uni-tuebingen.de
About GSK Biologicals
GlaxoSmithKline (GSK) – a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. GSK is the world’s leading vaccine company, with a portfolio that helps protect people throughout life and an innovative pipeline of 15 vaccines in development. Our vaccines help prevent illnesses such as hepatitis A and B, diphtheria, tetanus, whooping cough, measles, mumps, rubella, polio, pneumococcal disease, influenza, shingles and meningitis. At GSK, more than 17,000 people worldwide deliver around 2 million vaccine doses per day to people in more than 160 countries. GSK’s three global R&D centres in Rixensart (Belgium), Rockville (US), and Siena (Italy) are focused on discovering and developing novel vaccines across a range of pressing public health threats, concentrating on those which offer significant improvements over existing options or tackle diseases for which no vaccines yet exist. GSK is focused on addressing the burden of AMR, preventing infections to reduce society’s dependence on antibiotics. For such purpose, GSK is actively participating in the development of innovative AMR-relevant vaccines aimed at preventing infections where target pathogens are likely to drive antibiotic use and resistance. GSK is collaborating with external experts to expand and to strengthen the evidence-base on vaccines and AMR and is supporting the expansion of economic models for vaccines that account for the value of reduced antibiotic use.
Link to website:
www.gsk.com
About Pfizer
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products. Our global portfolio includes medicines and vaccines as well as many of the world’s best-known consumer health care products. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments, and local communities to support and expand access to reliable, affordable health care around the world. For more than 150 years, Pfizer has worked to make a difference for all who rely on us. The Patient Health Impact (PHI) – Health Economics and Outcomes group will contribute to the PrIMAVeRa project with contributors located in the Europe and United States. The goal of the group is to support the PrIMAVeRa initiative to generate the necessary evidence, develop the right model structure and conduct detailed cost-effective analysis.
Link to website:
www.pfizer.com
About Janssen
Janssen Vaccines & Prevention’s corporate headquarters and main R&D facilities are located in Leiden, The Netherlands. Janssen Vaccines & Prevention is part of the Janssen group of Pharmaceutical Companies of Johnson & Johnson. The company currently has several new vaccine candidates in the pipeline including vaccine candidates aimed at preventing respiratory infections (COVID-19, RSV, influenza), HIV and several pathogens of global concern (Ebola, polio), as well as vaccine candidates against diseases caused by Extraintestinal Pathogenic E. coli (ExPEC) and S.aureus that are linked to AMR (Anti-Microbial Resistance). In addition, the company is investing in platform technology development to support and extend the vaccine pipeline.
Link to website:
www.janssen.com
For PrIMAVeRa inquiries, please contact:
European Vaccine Initiative | Coordinating institution
www.euvaccine.eu
Project Leader
Irina Meln
Universitätsklinikum Heidelberg
Voßstraße 2, Geb. 4040
69115 Heidelberg, Germany
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 101034420. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution. https://www.imi.europa.eu/ |