Follow us on:
Mission and Vision
Non-tuberculous mycobacteria (NTM) cause lung diseases that look similar like the ones caused by Mycobacterium tuberculosis (MTB). NTM mainly affect people with weakened immune systems or patients with other lung diseases such as cystic fibrosis. NTM infections are caused by bacteria such as Mycobacterium avium complex and Mycobacterium abscessus, both of which are related to the TB-causing MTB bacteria. Cases of NTM disease are on the rise worldwide, and in regions where TB is largely under control, NTM is much more common than TB. Although treatments exist for the most common NTM infections, they do not always work well, and the complex treatment regimen can last as long as two years. Moreover, recent studies have shown that there are now cases of multi-drug resistant NTM.
The goal of RespiriNTM is to find new drug candidates that could be part of a new, more efficient drug regimen for NTM with a shorter treatment time. The team will focus on discovering inhibitors with a new mechanism of action that ideally will synergize with inhibitors of the respiratory pathway of the bacteria. In addition, the team will study the factors that allow NTM bacteria to survive in humans.
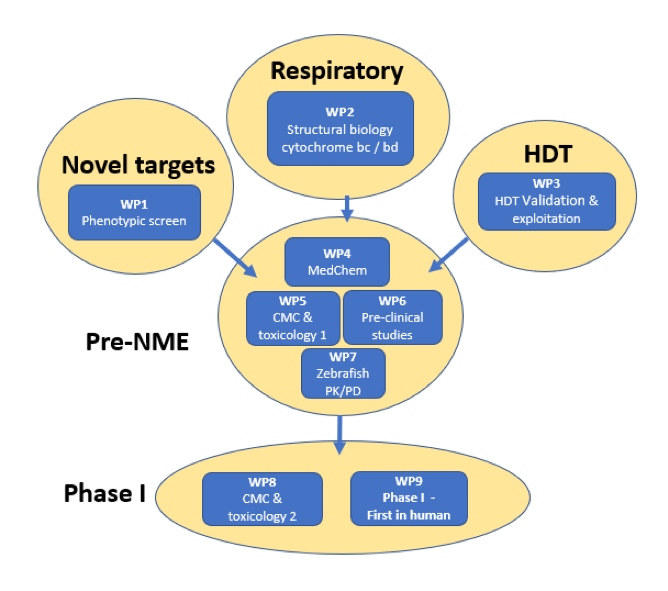
Strategy
RespiriNTM will perform a high-throughput inhibitor screen against Mycobacterium avium complex (MAC). In addition, the project team will perform a screening campaign to identify human factors that are essential for the survival of the bacterium in the human cell. Selected hits will be optimized through medicinal chemistry, while structures of target proteins bound to inhibitor will be determined to aid in this optimization.
Lead compounds will be screened for stability, toxicology, pharmacokinetics and dynamics as well as suitable delivery methods. After pre-clinical studies in animal models, First-In-Human trials will be undertaken as a milestone step towards final development of a novel antibiotic.
Governance
Publications
- Fernow J, Olliver M, Couet W, et al. The AMR Accelerator: from individual organizations to efficient antibiotic development partnerships. Nature Reviews Drug Discovery 2024. Published online September 23, 2024. doi:10.1038/d41573-024-00138-9. Green Open Access available through DiVA.
- Johansen MD, Spaink HP, Oehlers SH, Kremer L. Modeling nontuberculous mycobacterial infections in zebrafish. Trends in Microbiology. 2024;32(7):663-677. doi:10.1016/j.tim.2023.11.011
-
Kilinç G, Boland R, Heemskerk MT, et al. Host-directed therapy with amiodarone in preclinical models restricts mycobacterial infection and enhances autophagy. Subbian S, ed. Microbiology Spectrum. 2024;12(8). doi:10.1128/spectrum.00167-24
- Hu W, Koch BEV, Lamers GEM, Forn-Cuní G, Spaink HP. Specificity of the innate immune responses to different classes of non-tuberculous mycobacteria. Frontiers in Immunology. 2023;13:1075473. doi:10.3389/FIMMU.2022.1075473
-
Kilinç G, Walburg K v., Franken KLMC, et al. Development of Human Cell-Based In Vitro Infection Models to Determine the Intracellular Survival of Mycobacterium avium. Frontiers in Cellular and Infection Microbiology. 2022;12:872361. doi:10.3389/FCIMB.2022.872361
-
Kilinç G, Saris A, Ottenhoff THM, Haks MC. Host-directed therapy to combat mycobacterial infections*. Immunological Reviews. 2021;301(1):62-83. doi:10.1111/IMR.12951
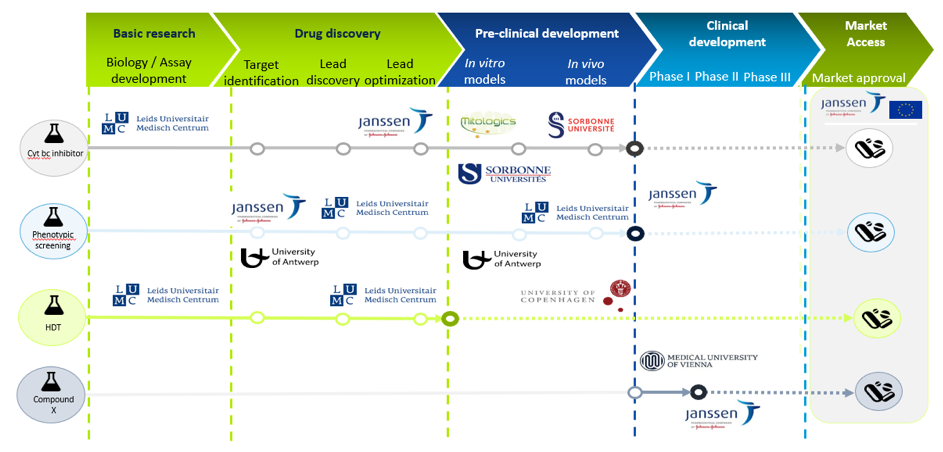
Project consortium
About BioVersys
BioVersys AG is a privately owned Swiss pharmaceutical company focusing on research and development of small molecules acting on novel bacterial targets with applications in Anti-Microbial Resistance (AMR) and targeted microbiome modulation. With the company’s award-winning TRIC technology we can overcome resistance mechanisms, block virulence production and directly affect the pathogenesis of harmful bacteria, towards the identification of new treatment options in the antimicrobial and microbiome fields. Our most advanced R&D programs are in preclinical development for nosocomial infections (hospital infections), and Tuberculosis in collaboration with GlaxoSmithKline (GSK) and a consortium of the University of Lille. BioVersys is located in the Technologiepark in the thriving biotech hub of Basel.
About Leiden University Medical Center (LUMC)
LUMC is a university medical center for research, education and patient care with a high-quality profile and a strong scientific orientation. It has a unique research practice, ranging from pure fundamental medical research to applied clinical research. Researchers at LUMC work together around 7 medical research profiles: Vascular and Regenerative Medicine; Immunity, Infection and Tolerance; Translational Neuroscience; Cancer Pathogenesis and Therapy; Ageing; Innovation in Health Strategy and Quality of Care; and Biomedical Imaging. LUMC is a center for medical innovation, committed to the advancement of health care and education in line with the latest international insights and standards – and aims to play a nationally and internationally recognized leading role in improving medicine and the quality of health care.
Contact:
Meindert Lamers (Project Coordinator) – m.h.lamers@lumc.nl
Tom Ottenhoff – T.H.M.Ottenhoff@lumc.nl
Marielle Haks – M.C.Haks@lumc.nl
About University of Antwerp
The University of Antwerp (UAntwerpen) is a fully accredited Belgian University with high standards of research and education. UAntwerpen is ranked 14th in the World, 7th in Europe among the “young” universities (less than 50 years of age) and 210th among all universities according to QS World University Rankings 2018. Although UAntwerpen is a mid-size university in Belgium, the CWTS Leiden Ranking in Natural Sciences ranks UAntwerpen 1st in Belgium in collaborative publications and 3rd in the category impact. The Uantwerpen is represented by 3 groups (LMPH, ORSY and UAMC) in RespiriTB and 2 groups (ORSY and UAMC) in RespiriNTM projects
Contact:
Koen Augustyns – koen.augustyns@uantwerpen.be
Bert Maes – bert.maes@uantwerpen.be
About Sorbonne University
Sorbonne Université hence becomes a fully multidisciplinary research-intensive university with three faculties: Humanities and Social Sciences, Medicine and Sciences & Engineering. With more than 53 400 students (among 10 200 international students), 4400 doctoral students and 6300 researchers, Sorbonne Universitéis a leading French university. It assists the researchers for all the administrative, financial and legal aspects of the research projects. In particular, the research officers ensure a follow up of the project results and their adequate exploitation and technology transfer.
The Sorbonne group is leading by Dr. Vincent Jarlier & Dr. Nicolas Veziris and Dr. Alexandra Aubry. The group brings to the consortium a large medical and biological expertise on mycobacteriology, including M. tuberculosis and non-tuberculous mycobacteria (NTM) of medical importance, as well as the vision of therapeutic applications. The team focuses mainly its activity on therapeutic aspects of tuberculosis and NTM infections: (a) study of new drugs, based on microbiological technics, molecular biology, biochemistry and structural biology; (b) in vivo activity and efficiency of new therapeutic regimens using various mouse models; (c) mechanisms of resistance in clinical strains and in vitro selected mutants, based on microbiological technics, molecular biology (sequencing, WGS…) and biochemistry.
https://www.sorbonne-universite.fr
Contact:
Alexandra Aubry – alexandra.aubry@sorbonne-universite.fr
Nicolas Veziris – nicolas.veziris@sorbonne-universite.fr
About MedUni Vienna
The Medical University of Vienna (briefly: MedUni Vienna) is one of the most traditional medical training and research facilities in Europe. With its 26 university hospitals, three clinical institutes, 12 theoretical medicine centres and numerous highly specialised laboratories, it is included among the most important cutting-edge research institutes of Europe in the area of biomedicine. The role of MUW in the programme will be to plan, perform and analyse clinical first in man studies in healthy subjects and studies in specific patient populations with all compounds developed by the consortium. The clinical assessment will cover safety and tolerability. After biochemical quantification of the pharmacokinetic samples in a certified laboratory, pharmacokinetic analysis will be performed to optimize preclinical models as well as PK/PD modelling in other work packages.
The Department of Clinical Pharmacology of the Medical University of Vienna is an academic institution which aims at providing expertise and special infrastructure on preclinical and clinical drug development with the ultimate goal to develop and evaluate innovative therapeutic interventions and diagnostic technologies. The department performs 30 phase 1 studies per year including several FIM studies. During the RespiriTB/NTM projects, the Department of Clinical Pharmacology of the Medical University will be represented by Prof. Markus Zeitlinger.
https://www.meduniwien.ac.at/web
Contact:
Markus Zeitlinger – markus.zeitlinger@meduniwien.ac.at
About University of Copenhagen
University of Copenhagen is the largest and oldest university in Denmark and one of the oldest researches and teaching institutions in Northern Europe. The 38.000 students and 9.700 employees (of which more than 5000 are researchers) work on four large campus areas in Copenhagen. The University consists of 6 faculties consisting of 36 departments and more than 200 research centers. The University of Copenhagen is ranked as #22 in Europe and #73 in the world, according to QS World University Rankings 2017.
UCPH group directed by Dr. Anette Müllertz and Prof. Thomas Rades will be responsible for the physico-chemical characterization of the lead compounds identified by the other partners and Jansen. This will include solubility and supersaturation propensity of the lead compounds. Based on the outcome, a formulation strategy will be formed. Drug delivery systems will be developed using physiological relevant in vitro models simulating the GI tract. Both lipid-based and amorphous delivery approaches will be considered for developing the most suitable formulation for increasing the bioavailability. Selected drug delivery systems will be tested for their pharmaco-kinetic profile in small animal models.
Contact:
Annette Müllertz – anette.mullertz@sund.ku.dk
Thomas Rades – thomas.rades@sund.ku.dk
About University Leiden
Leiden University was founded in 1575 and is one of Europe’s leading international research universities. It has seven faculties in the arts, humanities and sciences, spread over locations in Leiden and The Hague. The University has over 6,700 staff members and 28,130 students. Much of our research focuses on making the unknown known and extending the boundaries of existing fields of research. Our research has a strong disciplinary basis within a very broad range of scientific fields. The university has thirty research institutes, located in both Leiden and The Hague, each of which conducts disciplinary and interdisciplinary research. Our researchers maintain close contacts with one another other and with society across the boundaries of their institutes and faculties. They are guided by the highest ambitions of quality and integrity.
Leiden group leader, Prof. Herman P. Spaink is the experts in the use of cell biology and systems biology and pharmacology to test efficacy and pharmacokinetic properties of drugs against infectious disease. For this we use as well high throughput experimental approaches as theory development to translate data from different test systems. A major focus has been on testing antibiotics in tuberculosis disease models, with a main emphasis of the responses of the pathogens inside the host immune cells. This is highly relevant since pathogens have a very different response to drugs when they are inside the host as compared to the free-living state. One of our major systems models for tuberculosis progression is the use of zebrafish larvae for high throughput screening.
https://www.universiteitleiden.nl
Contact:
Herman Spaink – h.p.spaink@biology.leidenuniv.nl
About Mitologics
Mitologics SAS is an innovative French biotechnology company (SME) founded in 2009 by Dr. Annie Borgne-Sanchez, Dr. Nelly Buronand Mathieu Porceddu. The company is specialized in the detection of mitochondrial alterations for applications in toxicology and pharmacology. The team has a strong expertise in cell and mitochondrial biology, metabolism, apoptosis and cancer. The company won the “Concours national d’aideà la creation d’entreprisesde technologies innovantes” in 2009 and 2011, awarded by the French Ministry of Education and Research. Mitologics is member of the “Pôlede CompétitivitéMEDICEN Paris-Région” and of the French association of R&D service providers participating to innovative research programs in Life Sciences (AFSSI).
Mitologics directed by Dr. Annie Borgne-Sanchez will evaluate compounds provided by partners of the consortium for their mitochondrial toxicity risks on its screening platform (MiToxView®). Both acute and long-term mitochondrial toxicity will be studied on isolated rodent mitochondria (first stage screening) and hepatic differentiated cells (on optimized compounds). Mitologics has published several papers validating its screening assays to detect drug-induced mitochondrial damages and performed several studies for pharmaceutical and cosmetic industry.
Contact:
Annie Borgne-Sanchez – aborgne.sanchez@mitologics.com
About FFUND BV
FFUND is a young and ambitious Dutch SME that provides full-service consultancy in project management, assistance in development of exploitation strategies, active communication, dissemination and promotion activities, based on years of experience. We combine scientific expertise and in-depth knowledge of project management with translational science and life science entrepreneurship. FFUND is able to identify exploitable research results, and support scientist in defining the optimal strategy for valorisation. Moreover, FFUND is expert at securing the right (non-) dilutive funding for R&D projects.
During the RespiriTB/NTM projects, FFUND’s PRINCE qualified project manager, Dr. Mima Malcicka, will aid in management and developing dissemination strategies. Moreover, FFUND plans for success continuation of the technology by securing follow-up funding throughout the complete value chain from the early start, through controlled growth, until a successful exit. FFUND will ask the tough questions to clear up any ambiguities and spot the innovative strengths, ultimately leading successful commercialization of the RESPIRI-TB results.
Contact:
Ilina Bareja – ilina.bareja@ffund.nl
Former consortium partner
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853932. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution. https://www.imi.europa.eu/ |