Follow us on:
Mission and Vision
Tuberculosis (TB) remains a significant public health problem world-wide, resulting in an estimated 10 million new infections and 1.5 million deaths in 2018 alone. Increasing resistance of Mycobacterium tuberculosis (M.tb) to currently available anti-TB drugs threatens much of the progress that has been made toward the global goal of TB elimination over recent years.
Ethionamide (ETH) and prothionamide (PTH) are valuable drugs in the treatment of TB. However, due to their suboptimal bioactivation within the M.tb, high doses are required to achieve clinical efficacy and are typically associated with increasing adverse events, most commonly gastrointestinal intolerance and hepatotoxicity. This results in ETH/PTH not being able to express their full activity. Despite this, ETH/PTH are part of a second-line anti-TB agent for the treatment of multi-drug resistant (MDR)-TB. Small molecules acting on regulators of the ETH/PTH bioactivation pathways in M.tb were shown to both a) boost ETH/PTH activity and b) revert resistance to this antibiotic class. Interestingly, these small molecules (boosters) render ETH/PTH active also against a vast majority of isoniazid-resistant strains.
The specific objectives of this IMI project are to deliver one Phase II ready booster molecule, having a) completed preclinical CTA enabling studies and b) completed Phase I (SAD and MAD) for safety and PK evaluation in healthy volunteers. Ultimately, the results from this project will pave the way for the booster to be integrated into new, improved regimens to treat TB including MDR-TB. A small molecule booster in combination with lower ETH/PTH doses will deliver a better tolerated and more potent drug combination than standard doses of ETH alone, and this can massively impact the current TB treatment armamentarium and significantly improve both patient experience and treatment outcomes.
What’s behind TRIC-TB?
Strategy
The booster molecules act on M.tb transcription regulators, resulting in an increase of the bioactivation of ETH/PTH allowing to lower the doses of ETH of more than three-fold (based on in vivomouse data). Data show that activation of alternative bioactivation pathways by the boosters overcomes pre-existing resistance mechanisms to this antibiotic class and, interestingly, to a vast majority of isoniazid-resistant strains.
Making ETH/PTH more tolerated and significantly more potent on drug resistant and sensitive strains opens the scope of use for ETH also as a first line therapy against M.tb. Bacterial transcriptional regulators are relatively new targets in drug R&D that have not been exploited commercially to date. As a matter of fact, a small molecule targeting a bacterial transcriptional regulator will be the first example to be assessed in clinical trials and, hopefully, to reach the market.
Governance
The TRIC-TB consortium consists of an innovative SME, BioVersys, and an EFPIA member, GSK, a leading pharmaceutical company. Both partners combine rapid decision making with robust scientific testing in a highly collaborative environment.
This is something that has already proven to be very successful during the previous four years of collaboration between GSK and BioVersys and has resulted in the building of the TRIC-TB project to this point.
Publications
- Fernow J, Olliver M, Couet W, et al. The AMR Accelerator: from individual organizations to efficient antibiotic development partnerships. Nature Reviews Drug Discovery 2024. Published online September 23, 2024. doi:10.1038/d41573-024-00138-9. Green Open Access available through DiVA.
- Weston DJ, Thomas S, Boyle GW, Pieren M. Alpibectir: Early Qualitative and Quantitative Metabolic Profiling from a First-Time-in-Human Study by Combining 19 F-NMR (Nuclear Magnetic Resonance), 1 H-NMR, and High-Resolution Mass Spectrometric Analyses. Drug Metabolism and Disposition. 2024;52(8):858-874. doi:10.1124/dmd.124.001562
- Pieren M, Abáigar Gutiérrez-Solana A, Antonijoan Arbós RM, et al. First-in-human study of alpibectir (BVL-GSK098), a novel potent anti-TB drug. Journal of Antimicrobial Chemotherapy. 2024;79(6):1353-1361. doi:10.1093/jac/dkae107
Press release
BioVersys and GSK successfully completed the IMI2 programme, reaching a clinical milestone. Read more.
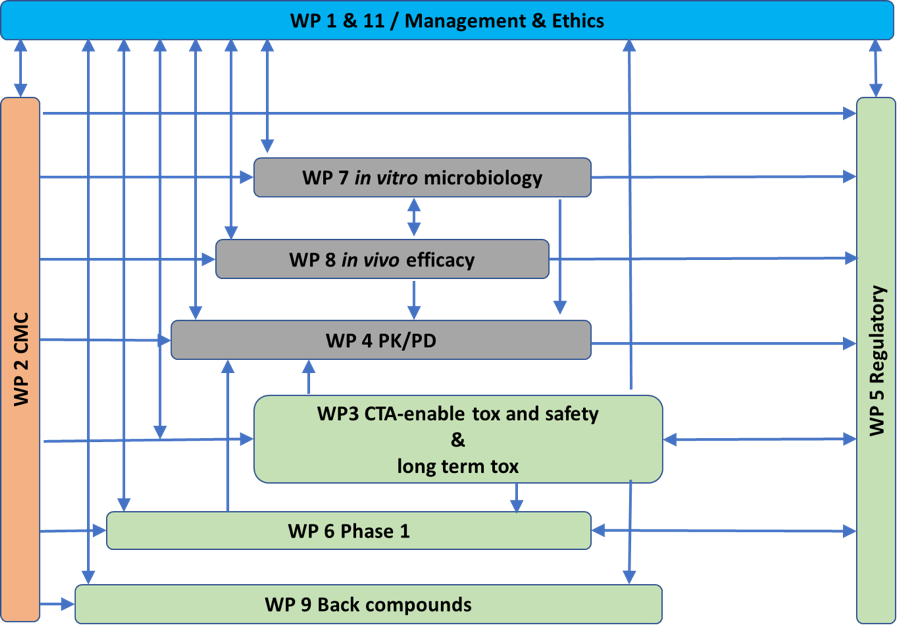
Project consortium
About GSK
GSK – is a science-led global healthcare company that researches and develops a broad range of innovative products. The R&D organization within the company has extensive expertise in designing, executing, analyzing and reporting clinical trials in many therapeutic areas, including infectious diseases. GSK is committed to reporting the results of clinical research that evaluate medicines and vaccines, irrespective of whether the outcomes are perceived to be positive or negative. GSK is an active member of several collaborations focused on developing new treatments for TB, including multi-drug resistant forms of the infection. In 2012, GSK Tres Cantos joined the TB Drug Accelerator Program – a partnership with several pharmaceutical and public-sector research institutions and the Bill & Melinda Gates Foundation, aiming to speed up the discovery of new medicines by collaborating on early stage research.
Contact:
David Barros – david.a.barros@gsk.com
Modesto Remuinan – modesto.b.remuinan@gsk.com
About BioVersys
BioVersys AG is a privately owned Swiss pharmaceutical company focusing on research and development of small molecules acting on novel bacterial targets with applications in Anti-Microbial Resistance (AMR) and targeted microbiome modulation. With the company’s award-winning TRIC technology we can overcome resistance mechanisms, block virulence production and directly affect the pathogenesis of harmful bacteria, towards the identification of new treatment options in the antimicrobial and microbiome fields. Our most advanced R&D programs are in preclinical development for nosocomial infections (hospital infections), and Tuberculosis in collaboration with GlaxoSmithKline (GSK) and a consortium of the University of Lille. BioVersys is located in the Technologiepark in the thriving biotech hub of Basel.
Contact:
Marc Gitzinger – marc.gitzinger@bioversys.com
Michel Pieren – michel.pieren@bioversys.com
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853800. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution. https://www.imi.europa.eu/ |