Follow us on:
Mission and Vision
The UNITE4TB (academia and industry united innovation and treatment for tuberculosis) project is a public-private partnership with representation from academic institutions, small- and medium-sized enterprises (SMEs), public organisations, and pharmaceutical companies.
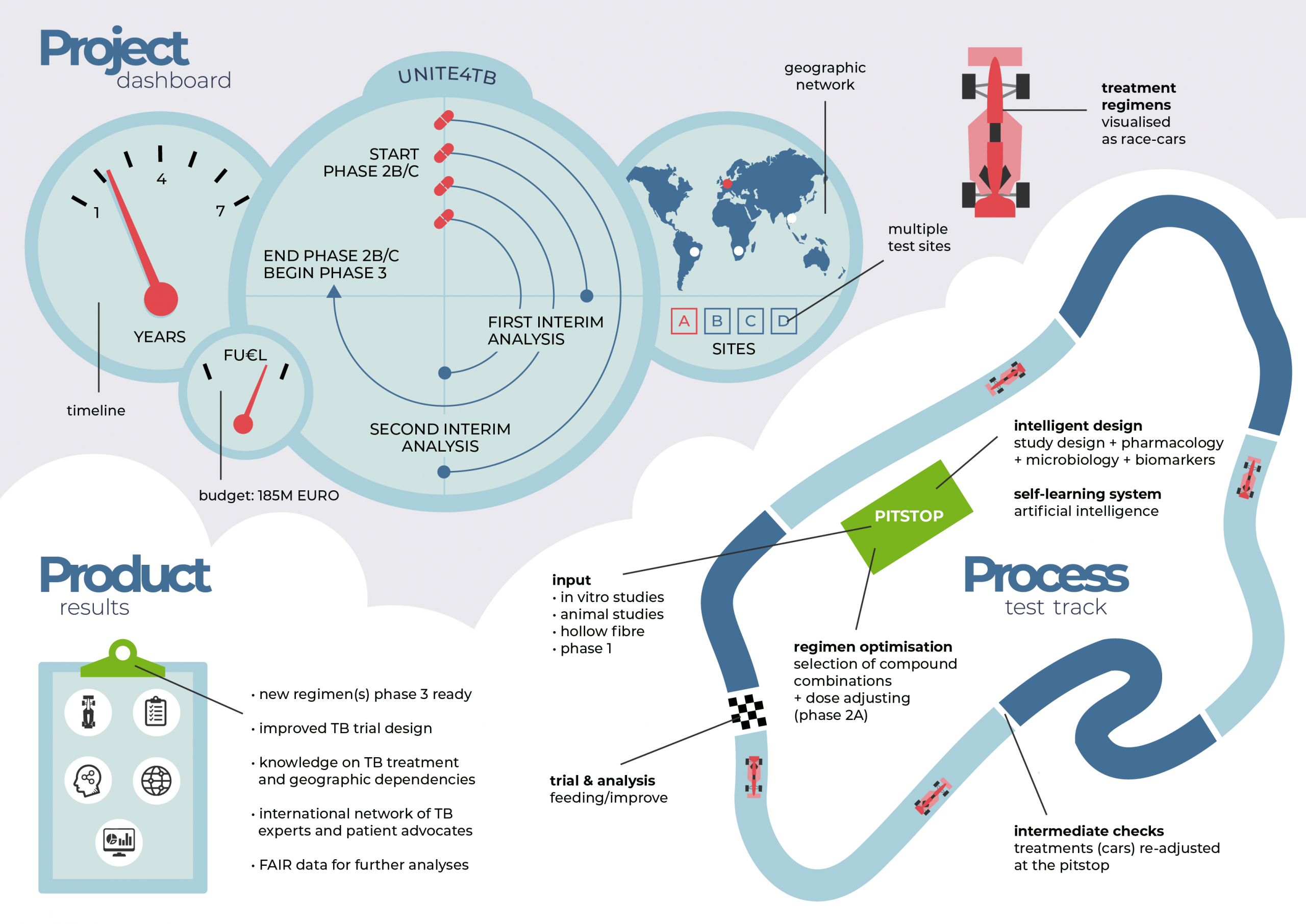
Over the next 7 years, the consortium will be active in approximately 40 trial sites on four continents (Europe, Asia, Africa and South America), with the goal of delivering novel phase 2 clinical trials that will accelerate the development of new TB drugs and regimens. Achieving this goal will facilitate fulfilment of one of the main unmet needs in the TB field: better-tolerated drug regimens of shorter duration that can be deployed to tackle tuberculosis across various drug-resistance patterns and co-morbidities.
Strategy
To address the challenge of developing new TB treatment regimens, UNITE4TB follows a concept that is analogous to a car racetrack. Through its EFPIA and Associated Partners UNITE4TB ensures access to the majority of the most innovative TB compounds. These compounds, the key car parts, will enter the pitstop when they are phase 2-ready and be incorporated into a new treatment regimen (the race car). Potential combination regimens will then be launched onto the racetrack (a clinical trial platform with 40 trial sites on four continents) to participate in the race to select the best car or treatment regimen.
New car parts (drugs) and cars (regimens) may enter the race when ready, making this a state-of-the-art adaptive trial design with conventional and new biomarkers of treatment success. Advanced pharmacokinetic and pharmacodynamic modelling techniques and artificial intelligence and machine learning techniques will be employed to select, test and deliver novel combination regimens with a high probability of success in subsequent phase 3 clinical trials.
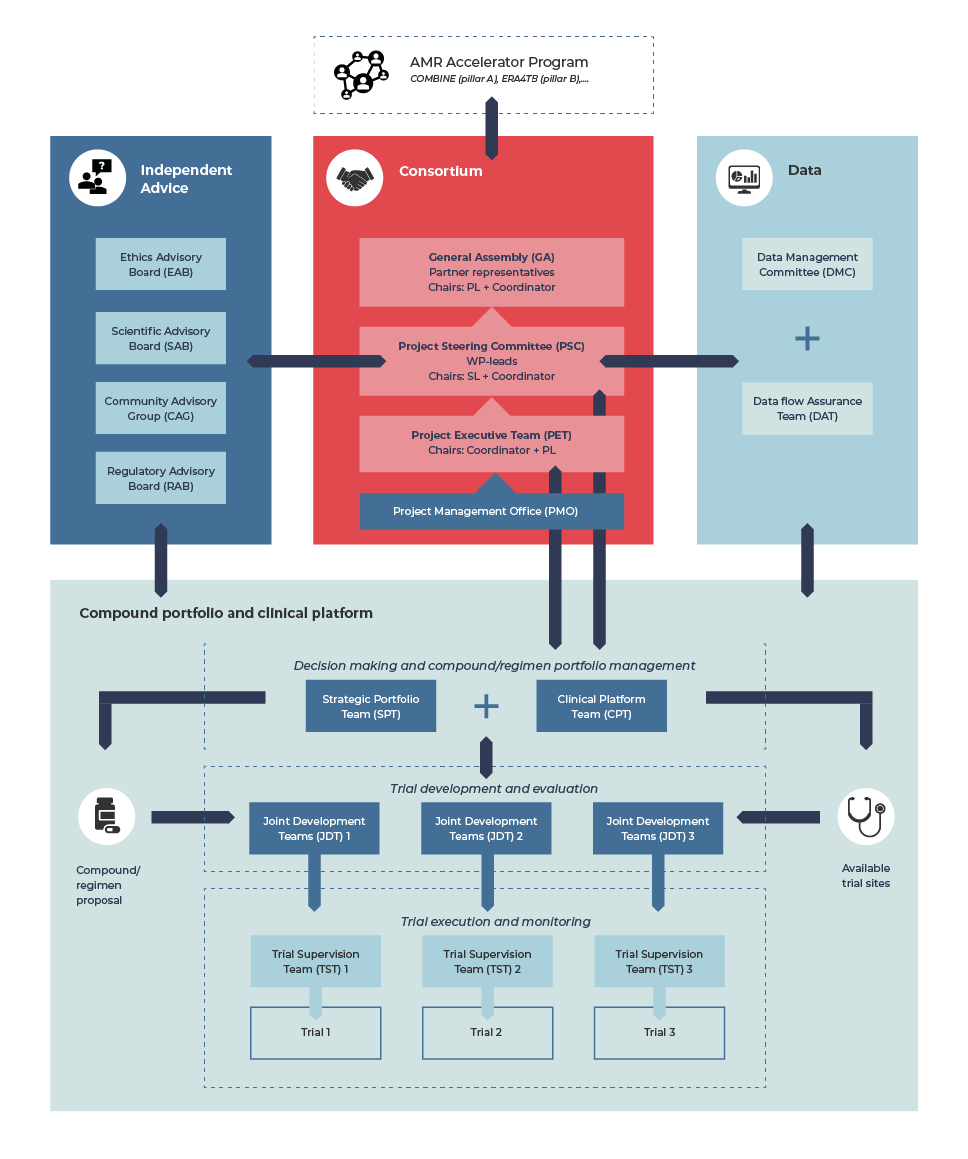
Governance
Publications
-
Behrens E, Wicha SG. Interoccasion variability in population pharmacokinetic models: identifiability, influence, interdependencies and derived study design recommendations. Journal of Pharmacokinetics and Pharmacodynamics. 2025;52(2):23. doi:10.1007/s10928-025-09966-7
- Dudnyk A, Lutchmun W, Duarte R, Lange C, Svensson EM. The importance of getting the dose right in the treatment of tuberculosis. Breathe. 2025;21(1):240177. doi:10.1183/20734735.0177-2024
- Lin Y, van der Laan LE, Karlsson MO, Garcia‐Prats AJ, Hesseling AC, Svensson EM. Model‐Informed Once‐Daily Dosing Strategy for Bedaquiline and Delamanid in Children, Adolescents and Adults with Tuberculosis. Clinical Pharmacology & Therapeutics. 2025;117(5):1292-1302. doi:10.1002/cpt.3536
- McClean M, Panciu TC, Lange C, Duarte R, Theis F. Artificial intelligence in tuberculosis: a new ally in disease control. Breathe. 2024;20(3):240056. doi:10.1183/20734735.0056-2024
- Schildkraut JA, Köhler N, Lange C, Duarte R, Gillespie SH. Advances in tuberculosis biomarkers: unravelling risk factors, active disease and treatment success. Breathe. 2024;20(3):240003. doi:10.1183/20734735.0003-2024
- Fernow J, Olliver M, Couet W, et al. The AMR Accelerator: from individual organizations to efficient antibiotic development partnerships. Nature Reviews Drug Discovery 2024. Published online September 23, 2024. doi:10.1038/d41573-024-00138-9. Green Open Access available through DiVA.
- Hoelscher M, Barros-Aguirre D, Dara M, et al. Candidate anti-tuberculosis medicines and regimens under clinical evaluation. Clinical Microbiology and Infection. 2024;30(9):1131-1138. doi:10.1016/j.cmi.2024.06.016
-
Gillespie SH, DiNardo AR, Georghiou SB, et al. Developing biomarker assays to accelerate tuberculosis drug development: defining target product profiles. The Lancet Microbe. 2024;0(0). doi:10.1016/S2666-5247(24)00085-5
-
Villa S, de Colombani P, Dall’Olio L, Gargioni G, Raviglione M. Towards comprehensive clinical trials for new tuberculosis drug regimens: policy recommendations from a stakeholder analysis. BMJ Global Health. 2024;9(4):e014630. doi:10.1136/BMJGH-2023-014630
-
Dufault SM, Crook AM, Rolfe K, Phillips PPJ. A flexible multi-metric Bayesian framework for decision-making in Phase II multi-arm multi-stage studies. Statistics in Medicine. 2024;43(3):501-513. doi:10.1002/SIM.9961
-
Mockeliunas L, Faraj A, van Wijk RC, et al. Standards for model-based early bactericidal activity analysis and sample size determination in tuberculosis drug development. Frontiers in Pharmacology. 2023;14:1150243. doi:10.3389/FPHAR.2023.1150243
-
Villar-Hernández R, Ghodousi A, Konstantynovska O, Duarte R, Lange C, Raviglione M. Tuberculosis: current challenges and beyond. Breathe. 2023;19(1). doi:10.1183/20734735.0166-2022
-
Saluzzo F, Adepoju VA, Duarte R, Phillips PPJ, Lange C. Treatment-shortening regimens for tuberculosis: updates and future priorities. Breathe. 2023;19(3):230028. doi:10.1183/20734735.0028-2023
-
Ness T, Van LH, Petermane I, et al. Rolling out new anti-tuberculosis drugs without diagnostic capacity. Breathe. 2023;19(2). doi:10.1183/20734735.0084-2023
-
Vasiliu A, Saktiawati AMI, Duarte R, Lange C, Cirillo DM. Implementing molecular tuberculosis diagnostic methods in limited-resource and high-burden countries. Breathe. 2022;18(4). doi:10.1183/20734735.0226-2022
-
Keutzer L, You H, Farnoud A, et al. Machine Learning and Pharmacometrics for Prediction of Pharmacokinetic Data: Differences, Similarities and Challenges Illustrated with Rifampicin. Pharmaceutics. 2022;14(8):1530. doi:10.3390/PHARMACEUTICS14081530
-
Heyckendorf J, Georghiou SB, Frahm N, et al. Tuberculosis Treatment Monitoring and Outcome Measures: New Interest and New Strategies. Clinical Microbiology Reviews. 2022;35(3). doi:10.1128/CMR.00227-21
-
Boeree MJ, Lange C, Thwaites G, et al. UNITE4TB: a new consortium for clinical drug and regimen development for TB. International Journal of Tuberculosis and Lung Disease. 2021;25(11):886-889. doi:10.5588/IJTLD.21.0515
-
Thacker V v., Dhar N, Sharma K, Barrile R, Karalis K, McKinney JD. A lung-on-chip model of early M. tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLife. 2020;9:1-73. doi:10.7554/ELIFE.59961
Project consortium
About Stichting Radboud Universitair Medisch Centrum (Radboudumc)
Stichting Radboud Universitair Medisch Centrum specializes in patient care, scientific research, teaching and training. Our mission is to have a significant impact on healthcare. We aim to be pioneers in shaping the healthcare of the future, in a person-centered and innovative way. In cooperation with partners, Radboud university medical center leads and coordinates Unite4TB.
Link to website:
www.radboudumc.nl/en/patient-care
About London School for Hygiene and Tropical Medicine (LSHTM)
The London School for Hygiene and Tropical Medicine is a global leader in public health, with cross-faculty interest in TB research and international high-impact clinical trials. LSHTM will participate in the overall academic leadership of the consortium as well as the implementation of the clinical trials through its network of TB clinical research sites in Asia.
Link to website:
www.lshtm.ac.uk
About University of Oxford
The University of Oxford department of Tropical Medicine & Global Health is collaborating in UNITE4TB via The Oxford University Clinical Research Unit (OUCRU) in Vietnam. OUCRU is a large-scale clinical and public health research unit with campuses in Ho Chi Minh City and Hanoi. OUCRU is hosted by the Hospital of Tropical Diseases (HTD) in Ho Chi Minh City, and the National Hospital for Tropical Diseases (NHTD) in Hanoi and is a Wellcome Africa and Asia Programme. OUCRU‘s ten-year vision is to have local, regional and global impact on health by leading a locally driven research programme on infectious diseases in Southeast Asia.
Link to website:
www.oucru.org
About Forschungszentrum Borstel, Leibniz Lungenzentrum
Forschungszentrum Borstel, Leibniz Lungenzentrum is a publicly funded foundation for pulmonary diseases research and clinical care. In UNITE4TB, the Forschungszentrum Borstel will participate in the coordination of clinical trials and will perform biomarker and pathogenomic research.
Link to website:
www.fz-borstel.de
About Lygature
Lygature, a not-for-profit foundation, acts as the independent mediator of the consortium, providing governance in terms of progress, finance and collaboration. Since 2006, Lygature has supported close to 100 public-private partnerships in the field of life sciences & health, including poverty-related diseases.
Link to website:
www.lygature.org
About Lancaster University
Lancaster University is a research-intensive university ranked in the UK’s top ten. Around 16,000 students from more than 100 countries choose to study at Lancaster because of our excellent reputation for research, teaching and student satisfaction. Lancaster is a truly global community with international collaborations all over the world and teaching partnerships in China, Malaysia, Ghana and Germany. In UNITE4TB, the Lancaster University team will co-lead the work on designing new clinical trials, creating computer simulations to evaluate these designs.
Link to website:
www.lancaster.ac.uk
About University College London
University College London, founded in 1826, is London’s leading multidisciplinary university, with more than 13,000 staff and 42,000 students from 150 different countries. Within UCL, UCL-TB is a broad and cross-disciplinary Tuberculosis research network that includes our partners throughout the world. Central to UCL-TB are the MRC CTU at UCL and the Centre for Clinical Microbiology (CCM) who work together to provide statistical and microbiological expertise for global TB clinical trials and research. In UNITE4TB, UCL will provide this same expertise in the delivery of TB clinical trials.
Link to website:
www.ucl.ac.uk
About TASK
Link to website:
www.task.org.za
About Vita-Salute San Raffaele University (UniSR)
The Vita-Salute San Raffaele University (UniSR), internationally recognized among the most prestigious universities, acts as coordinator of the microbiology work package for the academic side. UniSR will liaise with the other academic and industry partners involved in this task to provide guidance and technical support in all the microbiological aspects.
Link to website:
www.unisr.it
About Helmholtz Zentrum München
The Helmholtz Zentrum München is a research center with the mission to discover personalized medical solutions for the prevention and therapy of environmentally-induced diseases and promote a healthier society in a rapidly changing world. It investigates major diseases which develop from the interaction of lifestyle, environmental factors and personal genetic background, focusing particularly on diabetes mellitus, allergies and chronic lung diseases.
Link to website:
www.helmholtz-munich.de
About KNCV Tuberculosis Foundation (KNCV)
The KNCV Tuberculosis Foundation has been fighting TB since its establishment in 1903. Over these 118 years, the organization has acquired indispensable knowledge and experience in the field of effective TB prevention and care, resulting in significant contributions to global evidence generation, policy development and TB program implementation worldwide. KNCV will support the implementation of innovative patient support technologies such as digital adherence technologies throughout the project.
Link to website:
www.kncvtbc.org
About Critical Path Institute
In Critical Path Institute, Limited, is a wholly owned subsidiary of Critical Path Institute, an independent, non-profit established as a public-private partnership. C-Path, Ltd. will lead the data sharing workgroup for UNITE4TB and will assist with machine learning and artificial intelligence efforts. C-Path’s mission is to catalyse the development of new approaches that advance medical innovation and regulatory science, accelerating the path to a healthier world. C-Path, Ltd. EU is in Dublin, Ireland, and C-Path U.S. is in Tucson, Arizona.
Link to website:
www.c-path.eu
About the European Lung Foundation
The European Lung Foundation (United Kingdom) is a patient-led organisation that works internationally to bring patients and the public together with healthcare professionals to improve lung health and advance diagnosis, treatment, and care. ELF works with people from all over the world, including our volunteer patient network of more than 350 people and our patient organisation network with more than 200 respiratory organisations in Europe; working together with people living with more than 40 different lung conditions.
Link to website:
www.europeanlung.org
About Instituto de Saude Publica da Universidade do Porto (ISPUP)
Instituto de Saude Publica da Universidade do Porto (ISPUP), a private non-profit association, will lead the establishment of the new UNITE4TB consortium in the existing TB-ecosystem, with focus on the development of health care education and implement online methods of engagement with key stakeholder groups. Since 2015, ISPUP’s has a research group with the focus on Infectious Diseases Epidemiology, which studies diseases such as Mycobacterium tuberculosis, Human Immunodeficiency Virus, Hepatitis B and C.
Link to website:
www.ispup.up.pt
About the University of Liverpool
The University of Liverpool is a UK Russell Group University, active in Anti-infective pharmacology, Antimicrobial Resistance and Global Health research, which has led or participated in numerous related consortia, including PreDiCT-TB and PanACEA2.
Link to website:
www.liverpool.ac.uk
About Institut de Recherche Pour le Développement
Institut der Recherche pour le Développement, known in English as the French National Research Institute for Sustainable Development (IRD), is a French public research organisation working in partnership with low and middle-resource countries. IRD sets its priorities in line with the Sustainable Development Goals to steer global development and address the environmental, economic, social, and health challenges that affect the planet.
Link to website:
www.en.ird.fr
About the University of Hamburg
The University of Hamburg is the largest research and educational institution in Northern Germany and was awarded the status of a ‘University of Excellence’ in 2019. The Department of Clinical Pharmacy at the Institute of Pharmacy is devoted to optimization of anti-infective therapies.
Link to website:
www.chemie.uni-hamburg.de
About University of California San Francisco (UCSF)
The University of California San Francisco (UCSF) was established in 1864 and its School of Medicine is the oldest continuously operating medical school in the western US, is ranked number one in National Institutes of Health funding and is consistently ranked as one of the top five medical schools in the USA. UCSF received $647.8 million in NIH funding in 2018. UCSF’s mission is to advance health worldwide and has the collective faculty and staff to provide leadership, experience and expertise to implement the proposed project.
Link to website:
www.ucsf.edu
About TB Alliance
The TB Alliance is a not-for-profit product development partnership dedicated to the discovery, development and delivery of better, faster-acting and affordable tuberculosis drugs that are available to those who need them. Since 2000, TB Alliance has assembled the single largest pipeline of TB drug candidates and is driving equitable access to new treatment regimens.
Link to website:
www.tballiance.org
About FIND
FIND, the global alliance for diagnostics, seeks to ensure equitable access to reliable diagnosis around the world. We connect countries and communities, funders, decisionmakers, healthcare providers and developers to spur diagnostic innovation and make testing an integral part of sustainable, resilient health systems. We are working to save 1 million lives through accessible, quality diagnosis, and save US$1 billion in healthcare costs to patients and health systems. We are co-convener of the Access to COVID-19 Tools (ACT) Accelerator diagnostics pillar, and a WHO Collaborating Centre for Laboratory Strengthening and Diagnostic Technology Evaluation.
Link to website:
www.finddx.org
About University of Milano (UMIL)
The University of Milano is a research university devoted to education and research and is member of the League of European Research Universities (LERU) and founding member of the European Global Health Research Institutes Network (EGHRIN). At UMIL, the Centre for Multidisciplinary Research in Health Science participates in several projects on infectious diseases, including TB, like EU-PEARL.
Link to website:
www.unimi.it
About University St Andrews
The University St Andrews, founded in the 15th century, is Scotland’s first university and the third oldest in the English-speaking world. It is one of Europe’s most research-intensive seats of learning and one of the top-rated universities in Europe for research, teaching quality, and student satisfaction. In UNITE4TB, St Andrews will contribute to the improvements in speed and quality of tuberculosis clinical trials by researching new methods to monitor response to treatment.
Link to website:
About Uppsala University
Uppsala University, the country’s only Faculty of Pharmacy, has the scientific breadth required to develop a drug from laboratory to treatment. We perform a significant share of our work in interdisciplinary collaboration, holding key assignments in numerous international consortia. In UNITE4TB, Uppsala University provides expertise in pharmacometric modelling, AI and machine learning to understand the relationships between drug exposure, efficacy and safety.
Link to website:
About European Respiratory Society (ERS)
The European Respiratory Society (ERS) is one of the leading medical organisations in the respiratory field, with a growing membership spanning over 160 countries. ERS prioritises science, education and advocacy in order to promote lung health, alleviate suffering from disease and drive standards for respiratory medicine globally.
Link to website:
About TBnet
TBnet is a pan-European network consisting of over 600 health care professionals and scientists, aiming to promote quality of care for tuberculosis patients through training professionals, and conducting multi-country clinical and operational research. The network has a strong emphasis in addressing health-inequalities in TB services including access to diagnosis and treatment.
Link to website:
www.tbnet.eu
About GlaxoSmithKline Investigación y Desarrollo S L (GSK)
GlaxoSmithKline Investigación y Desarrollo S L will bring its expertise in Global Health and tuberculosis research to UNITE4TB as the consortium’s industry lead, mirroring its lead role with ERA4TB, which is investigating earlier stage assets as part of the IMI AMR Accelerator. GSK is a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer.
Link to website:
www.gsk.com
About Janssen Pharmaceutical NV
Janssen Pharmaceutical NV, The Pharmaceutical Companies of Johnson & Johnson, applies its expertise in the consortium to progress promising anti-TB compounds with the ambition of identifying a universal drug regimen, active against both drug susceptible and resistant TB. For over two decades, Janssen has been a committed partner in the global fight against TB including its development of bedaquiline.
Link to website:
www.jnj.com
About Otsuka Novel Products GmbH
Otsuka Novel Products GmbH is dedicated to finding innovative solutions to fight the global pandemic of tuberculosis (TB). As the European marketing authorization holder for Deltyba®, Otsuka Novel Products GmbH works in collaboration with the Otsuka group of companies, partners, non-governmental organisations and other stakeholders, to expand global access to Deltyba® and fight multidrug-resistant TB. ONPG is a part of Otsuka Pharmaceutical Company, Ltd., a subsidiary of Otsuka Holdings Co., Ltd. headquartered in Tokyo, Japan.
Link to website:
About Deutsches Zentrum für Infektionsforschung (DZIF)
Deutsches Zentrum für Infektionsforschung (DZIF), known in English as the German Center for Infection Research, has over 500 researchers from 35 institutions throughout Germany jointly developing new approaches for the prevention, diagnosis and treatment of infectious diseases. The aim is to translate research results into clinical practice quickly and effectively. With this, the DZIF paves the way for developing new vaccines, diagnostic agents and drugs to treat infections.
Link to website:
www.dzif.de
About LMU University Hospital Munich
LMU University Hospital Munich is one of the leading academic hospitals in Germany and a centre of high-end medicine, medical innovation and research. It is one of the largest university hospitals in Germany and Europe. In the field of tuberculosis, the main expertise of the Department of Infectious Disease and Tropical Medicine at LMU is the development and evaluation of novel drugs and diagnostics.
Link to website:
www.lmu-klinikum.de
UNITE4TB Project Leader
Dr Derek Sloan
Radboud University Medical Centre & University of St Andrews

| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 101007873. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA, Deutsches Zentrum for Infektionsforschung e.V. (DZIF), and Ludwig-Maximilians-Universität München (LMU). EFPIA contributes 50% of funding, whereas the contribution of DZIF and the LMU University Hospital Munchen has been granted by the German Federal Ministry of Research and Education. https://www.imi.europa.eu/ |