Follow us on:
Mission and Vision
The AMR Accelerator programme was created to accelerate the development of tools and treatments to fight drug-resistant bacteria and strengthen the scientific basis of AMR and TB research. 98 partners in nine different consortia are working to progress the development of new medicines to treat or prevent resistant bacterial infections. For the success of such consortia, capability building to support each project is of critical importance. The COMBINE project (“Collaboration for prevention and treatment of MDR bacterial infections”) has the following objectives:
- Provide coordination and support across the AMR Accelerator
- Establish an IT infrastructure for management, integration and analysis of combined data from across all AMR Accelerator projects, perform regular data management reviews, leverage best practices, create software specifications, review existing tools
- Facilitate communication and collaboration among AMR Accelerator partners, disseminate news and results, and increase visibility and outreach to key stakeholders in the field
- Share and analyse vaccine and antibacterial data to improve the design and analysis of clinical trials
- Improve the understanding of animal infection model reproducibility and translation to clinical efficacy
What’s behind COMBINE?
Strategy
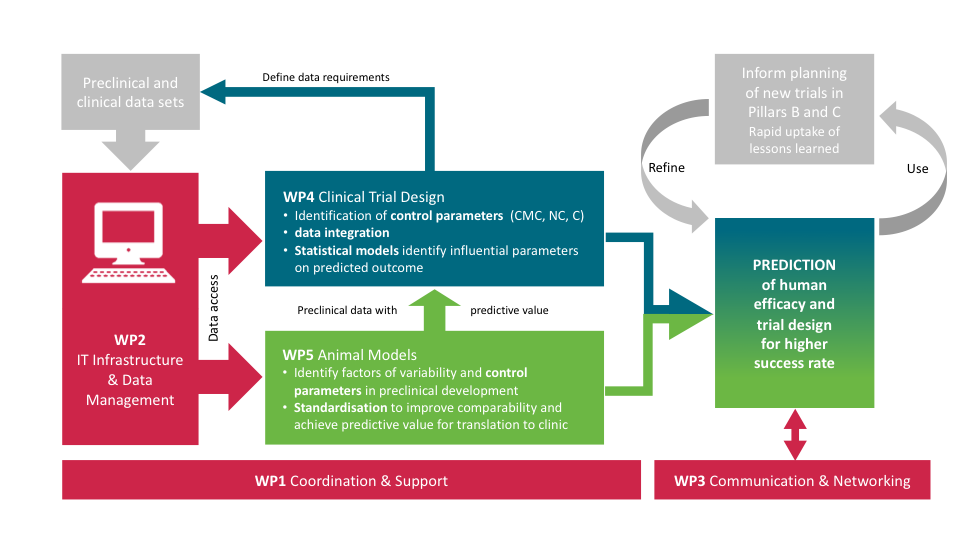
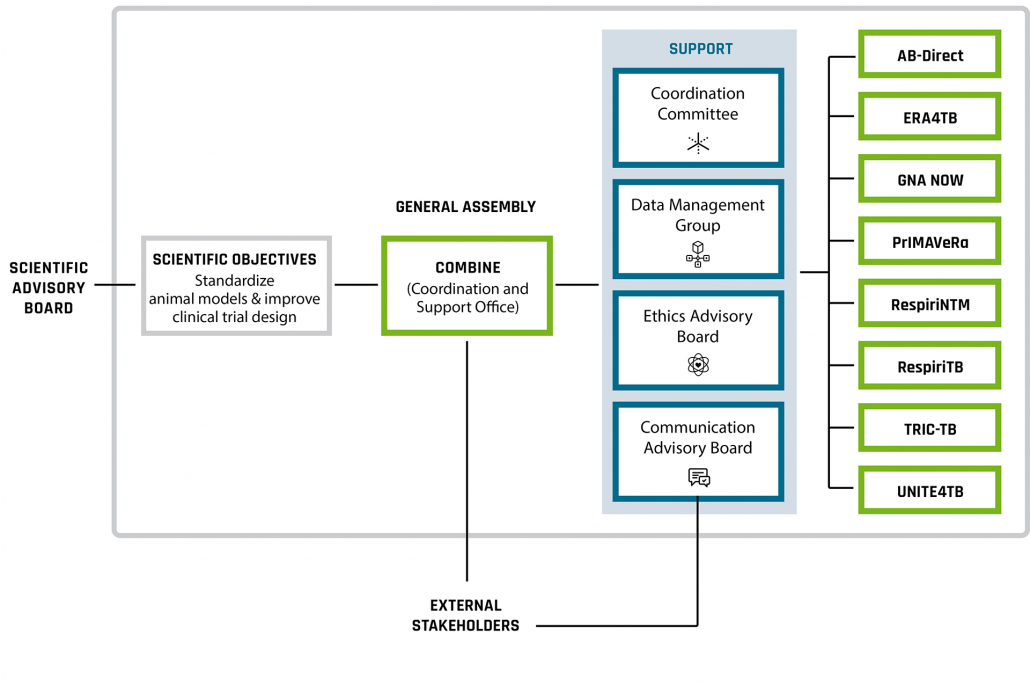
COMBINE has a coordination role (WP1, WP2, WP3) and a scientific mission (WP4, WP5)
Work Package 1: Coordination and Support
The Coordination and Support Office (CSO) coordinates support of the Accelerator projects. A number of Cross-Accelerator groups – a Data Management Group, a Coordination Committee, a Communication Advisory Board, and an Ethics Advisory Board – have been established as a resource for projects and will play key roles in overseeing progress in the AMR Accelerator.
Work Package 2: Guidelines, tools and infrastructure to support data and knowledge management
COMBINE will establish a robust data management process based on FAIR principles. As a basis, the ND4BB InfoCentre – which was set-up within TRANSLOCATION – will be used and extended. The software developed within COMBINE to enable cross-site access, and flexible handling of experimental documentation from early discovery through to development. It will support a wide range of data formats, from simple office documents and chemical structures to preclinical and clinical data sets, while retaining compatibility with all common operating systems. Documentation covering data governance, quality, access, and analysis procedures have already been developed and will be transferred to the programmes in the Accelerator.
Work Package 3: Communication, Dissemination and Networking
COMBINE will develop and implement communication and dissemination strategies and work to strengthen the interaction between AMR Accelerator participants and AMR stakeholders across the EU and globally. A strong corporate identity will be established, with logos and a joint website designed. COMBINE will coordinate the media outreach and social media activities, communicate results and attend conferences.
Work Package 4: Improving the design and analysis of clinical trials
COMBINE will help improve clinical trial design to increase the success rate of vaccine and antibiotic trials. COMBINE will also develop strategies and methodology for the analysis of clinical trial data. To achieve this, COMBINE experts will compare and evaluate non-clinical data or clinical patient-level data supplied by EFPIA partners, the BEAM Alliance, and others.
Work Package 5: Improved understanding of animal infection model reproducibility and translation to clinical efficacy
COMBINE will develop standardised protocols for rodent models, establish a reference strain collection and data bank, and provide a framework to make use of the results to improve translation to the clinic via mathematical modelling approaches. All animal models will use clinically relevant, well-defined Gram-negative strains. The standard protocols will ease harmonisation of non-clinical data and facilitate the establishment of standardized high-quality infection models.
Governance
In accordance with individual AMR Accelerator project requirements, COMBINE provides support in coordination, IMI-related project management, data management and communication.
Publications
Scientific papers
- Vera-Yunca D, Matias C, Vingsbo Lundberg C, Friberg LE. Model-based translation of the PKPD-relationship for linezolid and vancomycin on methicillin-resistant Staphylococcus aureus : from in vitro time–kill experiments to a mouse pneumonia model. Journal of Antimicrobial Chemotherapy. Published online May 9, 2025. doi:10.1093/jac/dkaf140
- Gadiya Y, Genilloud O, Bilitewski U, et al. Predicting Antimicrobial Class Specificity of Small Molecules Using Machine Learning. Journal of Chemical Information and Modeling. Published online February 23, 2025. doi:10.1021/ACS.JCIM.4C02347
- Fernow J, Olliver M, Couet W, et al. The AMR Accelerator: from individual organizations to efficient antibiotic development partnerships. Nature Reviews Drug Discovery 2024. Published online September 23, 2024. doi:10.1038/d41573-024-00138-9. Green Open Access available through DiVA.
- Arrazuria R, Kerscher B, Huber KE, et al. Expert workshop summary: Advancing toward a standardized murine model to evaluate treatments for antimicrobial resistance lung infections. Frontiers in Microbiology. 2022;13:988725. doi:10.3389/fmicb.2022.988725
- Arrazuria R, Kerscher B, Huber KE, et al. Variability of murine bacterial pneumonia models used to evaluate antimicrobial agents. Frontiers in Microbiology. 2022;13:988728. doi:10.3389/fmicb.2022.988728
-
Bekeredjian-Ding I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Frontiers in Immunology. 2020;11:533705. doi:10.3389/FIMMU.2020.01755
- Matias, C. S., Vera-Yunca, D., Friberg, L. E., Vingsbo Lundberg, C. (2025) Development of a Murine Pneumonia Model with Methicillin-resistant Staphylococcus aureus (MRSA) to Evaluate Antibiotic Treatments, presented at the ESCMID Global 2025, Vienna, Austria.
- Stojkov, I., Hofner, B. (2025) Exploring the Association Between Study Characteristics and Post-Vaccine Immunogenicity for C. diff: Data-Driven Analyses of Two Vaccine Trials, presented at the 9th AMR Conference, Basel, Switzerland.
- Fernow, J., Robijns, C., Vingsbo-Lundberg, C., Deroose, F., Sjöquist, M. (2024) Communicating about animal research in antibiotics drug development, presented at the Uppsala Antibiotic Days, Sweden.
- Vera-Yunca, D., Friberg, L. E. (2024) A multistate model for the analysis of clinical outcomes in nosocomial pneumonia patients, presented at the ESCMID Global 2024, Barcelona, Spain.
- Vera-Yunca, D., Friberg, L. E. (2024) A multistate model for the analysis of clinical outcomes in nosocomial pneumonia patients, presented at the thirty-second PAGE Meeting, Rome, Italy 2024.
- Gadiya, Y., Zaliani, A., Gribbon, P. (2024) Prediction of anti-bacterial class specificity of compounds using machine learning, presented at the 8th AMR Conference, Basel, Switzerland.
- Kerscher, B., Arrazuria, R., Vingsbo Lundberg, C., Hansen, J. U., Hoover, J. L., Sordello, S., Gribbon, P., Hughes, D., Friberg, L. E., Bekeredjian-Ding, I. (2024) The COMBINE Preclinical Bacterial Strain Repository (PBSR): a new resource for preclinical lung infection models to assess antibiotic efficacy, presented at the 8th AMR Conference, Basel, Switzerland.
- Stojkov, I., Marchioro, L., Hue Kästel, P. K., Kerscher, B., Hofner, B., Bekeredjian-Ding, I. (2024) Exploring challenges in vaccine development for ESCAPE pathogens: a study of regulatory interactions between developers and regulators, presented at the 8th AMR Conference, Basel, Switzerland.
- Vingsbo Lundberg, C., Hansen, J. U., Vera, D., Arrazuria, R., Kerscher, B., Hoover, J. L., Sordello, S., Bekeredjian-Ding, I., Hugues, D., Friberg, L. E. (2024) A standard protocol for the murine pneumonia model to investigate antibiotic treatment effect of MDR bacteria infections, presented at the 2024 Gordon Research Conference on Innovative Approaches in Antibacterial Research and Development to Fight the Antibiotic Resistance Crisis, Ventura, United States.
- Vingsbo Lundberg, C., Hansen, J. U., Vera, D., Arrazuria, R., Kerscher, B., Hoover, J. L., Sordello, S., Bekeredjian-Ding, I., Hughes, D., Friberg, L. E. (2023) Evaluation of a standard protocol for the murine pneumonia model to investigate antibiotic treatment effect of infections with MDR Gram negative bacteria, presented at the 2023 Joint ESCMID/ASM Conference, Boston, US.
- Stojkov, I., Marchioro, L., Hue Kästel, P. K., Hofner, B., Bekeredjian-Ding, I. (2023) Assessing regulatory communications in vaccine development against emerging pathogens: A pilot study, presented at the 2023 Fraunhofer CIMD Summer School, Leipzig, Germany.
- Vera-Yunca, D., Friberg, L. E. (2023) A translational semi-mechanistic pharmacokinetic-pharmacodynamic framework to design animal studies: Application to linezolid and vancomycin, presented at 31st PAGE Meeting 2023, Palexco A Coruña, Spain.
- Hansen, J. U., Arrazuria, R., Kerscher, B., Hoover, J. L., Vingsbo Lungberg, C., Sordello, S., Aranzana-Climent, V., Bekeredjian-Ding, I., Hughes, D., Gribbon, P., Friberg, L. E. (2022) A standard protocol for the murine pneumonia model to evaluate treatments for AMR lung infections, presented at the 2022 ESCMID/ASM Joint Conference, Dublin, Ireland, and at the 7th AMR Conference, Basel, Switzerland.
- Marchioro, L., Huber, K. E., Hofner, B., Kerscher, B., Arrazuria, R., Bekeredjian-Ding, I. (2022) Recurring problems and mitigation strategies in the development of monoclonal antibodies against AMR pathogens: a report from the COMBINE expert workshop, presented at the 2022 ESCMID/ASM Joint Conference, Dublin, Ireland.
- Gadiya, Y., Arrazuria, R., Hansen, J. U., Welter, D., Xu, F., Rocca-Serra, P., Juty, N., Gribbon, P. (2022) BPO: the Bioassay Protocol Ontology, a semantic approach to elevate reusability of AMR-related drug discovery data, presented at the 2022 ESCMID/ASM Joint Conference, Dublin, Ireland.
- Vingsbo Lundberg, C., Arrazuria, R., Kerscher, B., Huber, K. E., Hoover, J. L., Hansen, J. U., Sordello, S., Renard, S., Aranzana-Climent, V., Hughes, D., Gribbon, P., Friberg, L. E., Bekeredjian-Ding, I. (2022) A standardized murine pneumonia model to evaluate antibiotic treatments, presented at the Gordon Research Conference on Disruptive Antibiotics and Non-Antibiotic Therapies to Combat Drug-Resistant Bacterial Infections, Lucca, Italy.
- Marchioro, L., Huber, K. E., Hofner, B., Kerscher, B., Arrazuria, R., Bekeredjian-Ding, I. (2022) Recurring issues in the development of vaccines against AMR infections: results from the COMBINE vaccine expert workshop, presented at the 6th AMR Conference, Basel, Switzerland.
Presentations
- Marchioro, L. (2023) Improving clinical trials for candidate vaccines against antimicrobial resistance (AMR) infections: perspectives from the COMBINE project, delivered at the 2023 Zoonoses Conference, Berlin, Germany.
- Arrazuria, R. (2023) Standardization of the murine pneumonia model, delivered at the 7th AMR Conference on Basel, Switzerland.
- Gribbon, P. (2022) Common data resources to aid antibiotic drug discovery, delivered at the 2022 ASM/ESCMID Joint Conference, Dublin, Ireland.
- Vingsbo Lundberg, C. (2022) Challenges of in vivo studies to support pre-clinical to clinical translation, delivered at the 2022 ASM/ESCMID Joint Conference, Dublin, Ireland (recording available).
- Hoover, J. (2022) Improving animal models and preclinical-to-clinical translation, delivered at the 6th AMR Conference, Basel, Switzerland.
- Marchioro, L. (2022) Vaccines (and More) Against Antimicrobial Resistant Infections: The COMBINE Project (Part of the IMI AMR Accelerator), delivered at the 2022 Vaccine Development Workshop of the German Centre for Infection Research, online.
- Marchioro, L. (2022) Recurring issues in the development of vaccines against antimicrobial resistant (AMR) infections: results from the COMBINE vaccine expert workshop, delivered at the Annual Meeting of the 2022 Vaccine Working Group of the German Society for Immunology 4th AMR Conference, online.
- Karlén, A. (2021) The AMR Accelerator, delivered at the 5th AMR Conference, online (recording available).
- O’Dwyer, K. (2020) IMI AMR Accelerator – Structure and Strategy, delivered at the 51st UNION World Conference on Lung Health, online.
- Somers, G. (2020) IMI AMR Accelerator – public-private partnership for prevention and treatment of MDR bacterial infections, delivered at the 4th AMR Conference, online.
On-camera Interviews
Anders Karlén, COMBINE Project Coordinator, Uppsala University
Philip Gribbon, COMBINE Data Manager, Fraunhofer ITMP
Claus Stie Kallesøe, COMBINE Data Management Support, grit42
Coordination and Support Office
Anders Karlén
Uppsala University
Coordinator
Tim Miles
GlaxoSmithKline
Project Lead
Marie Olliver
Uppsala University
Alliance Manager
Anna Lobell
Uppsala University
Finance Manager
Frédéric Peyrane
BEAM Alliance
Outreach Manager
Tolganay Kabdullayeva
BIOCOM Interrelations GmbH
Communication Manager
Frederik Deroose
Connecting Pharma
Chair of Scientific Interest Groups
Sophie Lagrange
Evotec
Alignment Coordinator
Rogerio Martins
Evotec
Data Manager
Philip Gribbon
Fraunhofer ITMP
Data Manager
Anneleen Beckers
Janssen
Alignment Coordinator
Josepine Fernow
Uppsala University
Communication Strategist
Diarmaid Hughes
Uppsala University
Scientific Advisor
Benjamin Hofner
Paul-Ehrlich-Institut
Clinical trials
Igor Stojkov
Paul-Ehrlich-Institut
Clinical trials
Lena Friberg
Uppsala University
Animal models
Jennifer Hoover
GlaxoSmithKline
Animal models
Project consortium
About BEAM Alliance
Since its inception in 2016, the BEAM Alliance (Biotech companies from Europe innovating in Anti-Microbial resistance research) has succeeded in uniting European SMEs working in the field of AMR. The BEAM Alliance now represents over 60 European SMEs that are collectively developing over 140 new, diversified R&D projects focused upon prevention, diagnosis and cure of microbial infections. The goal of the BEAM Alliance is to promote and maintain awareness of SME-driven innovation in the field. The BEAM Alliance also supports policymakers in understanding economic business models around AMR, highlighting the specific needs of SMEs and incentives required to develop innovative diagnostics and treatments. Collaborating with the existing community of global stakeholders, the BEAM Alliance is actively working to curb the AMR threat in a sustainable manner.
About BIOCOM
BIOCOM is synonymous with professional communication skills and a wide range of specialist media for the life sciences. Content-driven and results-oriented since 1986.
For us, scientific ability goes hand in hand with sound communication skills – either as a double qualification offered by our individual employees or combined together in project teams. In addition, we offer a diverse spectrum of services, which is important for our customers. Alongside our contract work, we have also been successful in the market with our own high-quality products for more than 30 years: we are unparalleled in life sciences communication.
About Connecting Pharma
The management team at Connecting Pharma has in-depth experience in drug discovery. Before joining Connecting Pharma, the founder has built his career in Janssen Pharmaceutica (part of Johnson & Johnson) where he developed both scientific and managerial experience. He has been a key contributor in discovery programmes and is an inventor of multiple New Molecular Entities in the field of Anti-Infectives, Inflammation, Neuroscience and Oncology.
Connecting Pharma has a strong and strategic partnership with Scientist.com, the world’s largest online marketplace for medical research and is instrumentally involved in the development and implementation of the Scientist.com expansion strategy worldwide.
About Evotec
Evotec SE has built a strong, productive and successful business model serving as a partner to a variety of organisations (pharma and biotech companies, academic groups, foundations) to rapidly and efficiently advance assets from the discovery space to filing INDs (INDiGO) and on to early clinical development across multiple therapeutic areas. With the acquisition of Sanofi’s infectious diseases unit adding to the company’s already existing ID capacity, Evotec can serve as a key strategic partner for infectious diseases R&D. An internal team of the infectious diseases unit is dedicated to drug discovery and development, including in vitro and in vivo pharmacology, chemistry, development, and project management. Evotec will contribute to the enabling platforms by providing medicinal chemistry and design, in vitro and in vivo microbiological efficacy data, safety and ADME data. It will analyse PK/PD relationships, decipher modes of action, carry out CMC and fermentation and support clinical studies and modelling.
About Fraunhofer ITMP
The Fraunhofer-Gesellschaft is the leading organisation for applied research in Europe. Its research activities are conducted by 69 institutes and it employs a staff of 24,000; with an annual research budget of 2.1 billion euros. Fraunhofer Institute for Translational Medicine and Pharmacology ITMP is participant in COMBINE and contributes their cross-domain expertise in drug discovery and life science informatics. The Fraunhofer Institute ITMP, with 520 employees, conducts research in the field of applied life sciences from a molecular level to entire ecosystems.
Within ITMP, the Translational Medicine department develops new approaches for the diagnosis and treatment of diseases that are inadequately understood or controlled. The Department is involved in identification, functional characterization and validation of targets which play a role in human health, disease and toxicity.
About grit42
We’re grit42 from Copenhagen and we’ve been fixing data workflows since the Space Odyssey. Our core expertise is digital laboratory infrastructure, enabled by our scientific data management platform with user-friendly day-to-day lab workflows, as well as handling compound and sample logistics. By streamlining data across all the different pre-clinical drug discovery phases and integrating with all kinds of equipment, we facilitate structured and tagged quality data. In addition, the platform also features direct comparisons of data across experiments, links the data to other types of data, and it enables you to apply advanced analytics, including artificial intelligence (AI).
About GSK
GSK is a science-led global healthcare company that researches and develops a broad range of innovative products. The R&D organization within the company has extensive expertise in designing, executing, analyzing and reporting clinical trials in many therapeutic areas, including infectious diseases. GSK is committed to reporting the results of clinical research that evaluate medicines and vaccines, irrespective of whether the outcomes are perceived to be positive or negative. GSK is an active member of several collaborations focused on developing new treatments for TB, including multi-drug resistant forms of the infection. In 2012, GSK Tres Cantos joined the TB Drug Accelerator Program – a partnership with several pharmaceutical and public-sector research institutions and the Bill & Melinda Gates Foundation, aiming to speed up the discovery of new medicines by collaborating on early stage research.
About Janssen
At Janssen, we’re creating a future where disease is a thing of the past. We’re the Pharmaceutical Companies of Johnson & Johnson, working tirelessly to make that future a reality for patients everywhere by fighting sickness with science, improving access with ingenuity, and healing hopelessness with heart. We focus on areas of medicine where we can make the biggest difference: Cardiovascular & Metabolism, Immunology, Infectious Diseases & Vaccines, Neuroscience, Oncology, and Pulmonary Hypertension. We have a longstanding commitment to develop and responsibly deploy innovative technologies and treatments to combat the growing threat of antimicrobial resistance (AMR) on multiple fronts. Follow us at @JanssenGlobal.
About Paul-Ehrlich-Institut (PEI)
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, in Langen near Frankfurt/Main, is a senior federal authority reporting to the Federal Ministry of Health (Bundesministerium für Gesundheit, BMG). It is responsible for the research, assessment, and marketing authorisation of biomedicines for human use and immunological veterinary medicinal products. Its remit also includes the authorisation of clinical trials and pharmacovigilance, i.e. recording and evaluation of potential adverse effects. Other duties of the institute include official batch control, scientific advice and inspections. In-house experimental research in the field of biomedicines and life science form an indispensable basis for the manifold tasks performed at the institute. The Paul-Ehrlich-Institut, with its roughly 800 members of staff, also has advisory functions nationally (federal government, federal states (Länder)), and internationally (World Health Organisation, European Medicines Agency, European Commission, Council of Europe etc.).
About Statens Serum Institut (SSI)
Statens Serum Institut (SSI) is under the auspices of the Danish Ministry of Health. Our main duty is to ensure preparedness against infectious diseases and biological threats as well as control of congenital disorders. SSI has state-of-the-art laboratories and animal facilities and has extensive experience of designing, planning and execution of in vivo efficacy evaluation of antimicrobial compounds.
About Uppsala University
Uppsala University is the Nordic region’s oldest university – founded in 1477 – and is ranked among the top 100 universities in the world. There are more than 40,000 students here and they are seen, heard and make their mark everywhere. The University’s nearly 5,000 researchers and teachers conduct world-leading research and offer a seemingly endless number of courses.
Among the University’s alumni there are 15 Nobel Prize laureates, of which 8 received their prizes for discoveries made during their time at Uppsala University. Carl Linnaeus, Anders Celsius and Olof Rudbeck the Elder are a few examples of prominent scientists in Uppsala University’s history.
COMBINE Coordinator
Prof. Dr. Anders Karlén
Department of Medicinal Chemistry
Uppsala University
751 23 Uppsala, Sweden
COMBINE Project Lead
Tim Miles
GlaxoSmithKline Investigación y Desarrollo SL
C. de Severo Ochoa 2
28760 Tres Cantos
Madrid, Spain
COMBINE Communication
Tolganay Kabdullayeva
BIOCOM Interrelations GmbH
Jacobsenweg 61
13509 Berlin, Germany
Phone: +49 30 2649 2160
| This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 853967. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA companies’ in kind contribution. https://www.imi.europa.eu/ |