 https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2026-01-19 08:00:542026-01-22 12:37:25The COMBINE pneumonia model
https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2026-01-19 08:00:542026-01-22 12:37:25The COMBINE pneumonia modelThe AMR Accelerator Results
The AMR Accelerator Public Webinars feature critical discussions on AMR research. Hosted by experts across nine collaborative projects and the AMR ecosystem, these sessions offer valuable insights into the future of antimicrobial resistance solutions.
Discover all the recordings and open-access materials to join innovative conversations in AMR research.
The AMR Accelerator projects have produced a wide range of research and decision-making tools, starting from data templates and ontologies to infection models, strain repositories, pharmacokinetic models, assay protocols, and clinical trial networks.
Delve into the resources designed to support researchers and policymakers in advancing AMR research.
Find the latest AMR Accelerator publications showcasing cutting-edge AMR research, from novel therapeutics to AI-driven approaches.
Explore all articles via open-access links to gain insights and follow the latest scientific advances.
The AMR Accelerator Project Portfolio is an interactive overview of therapeutics, detailing programmes, asset owners, and development stages.
Access the detailed project information and track progress from discovery to clinical phases.
Latest news from the AMR Accelerator Projects
 https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2026-01-19 08:00:542026-01-22 12:37:25The COMBINE pneumonia model
https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2026-01-19 08:00:542026-01-22 12:37:25The COMBINE pneumonia model https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 08:50:072025-11-18 10:57:24AMR Accelerator Calls for Data
https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 08:50:072025-11-18 10:57:24AMR Accelerator Calls for Data https://amr-accelerator.eu/wp-content/uploads/2025/11/GNA-NOW-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 07:55:122025-11-17 16:14:06Pedro Henrique Quintela: Translating Discovery to Impact
https://amr-accelerator.eu/wp-content/uploads/2025/11/GNA-NOW-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 07:55:122025-11-17 16:14:06Pedro Henrique Quintela: Translating Discovery to Impact https://amr-accelerator.eu/wp-content/uploads/2025/11/GNA-NOW-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 07:52:362025-11-17 16:13:48Marie Attwood: Advancing In Vitro Modelling in AMR Research
https://amr-accelerator.eu/wp-content/uploads/2025/11/GNA-NOW-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-11-18 07:52:362025-11-17 16:13:48Marie Attwood: Advancing In Vitro Modelling in AMR Research https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-10-13 11:13:102025-11-05 11:14:4513/10/25 – PrIMAVeRa partners at 6th ESCMID Conference on Vaccines
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-10-13 11:13:102025-11-05 11:14:4513/10/25 – PrIMAVeRa partners at 6th ESCMID Conference on Vaccines https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-10-09 11:09:322025-11-05 11:10:3309/10/25 – UNITE4TB Expert Interview Series Complete
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-10-09 11:09:322025-11-05 11:10:3309/10/25 – UNITE4TB Expert Interview Series Complete https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-09-30 11:07:282025-11-05 11:08:5130/09/25 – UNITE4TB Flagship Trial Launches Wave 2 Recruitment
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-09-30 11:07:282025-11-05 11:08:5130/09/25 – UNITE4TB Flagship Trial Launches Wave 2 Recruitment https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-09-26 11:11:092025-11-05 11:12:3726/09/25 – Latest ERA4TB Webinar on Tackling AMR Through Policy and Partnerships
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-09-26 11:11:092025-11-05 11:12:3726/09/25 – Latest ERA4TB Webinar on Tackling AMR Through Policy and Partnerships https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-08-22 10:54:382025-09-10 10:55:4422/08/25 – PrIMAVeRa results selected for EU Innovation Radar Platform
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-08-22 10:54:382025-09-10 10:55:4422/08/25 – PrIMAVeRa results selected for EU Innovation Radar Platform https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-23 15:12:202025-09-10 10:51:4423/07/25 – UNITE4TB Flagship Trial Completes First Wave Recruitment
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-23 15:12:202025-09-10 10:51:4423/07/25 – UNITE4TB Flagship Trial Completes First Wave Recruitment https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-17 15:11:292025-09-10 10:52:2517/07/25 – UNITE4TB Expert Interview Series
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-17 15:11:292025-09-10 10:52:2517/07/25 – UNITE4TB Expert Interview Series https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-09 15:15:332025-09-10 10:54:1409/07/25 – 4th Scientific advisory committee meeting
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-07-09 15:15:332025-09-10 10:54:1409/07/25 – 4th Scientific advisory committee meeting
13/06/25 – New publication by PrIMAVeRa on systematic review of existing AMR transmission models
 https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-27 16:03:182025-06-03 16:04:1827/05/25 – UNITE4TB Annual Meeting 2025
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-27 16:03:182025-06-03 16:04:1827/05/25 – UNITE4TB Annual Meeting 2025 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-06 16:10:392025-06-03 16:11:4906/05/25 – ERA4TB will host public key-note speeches at annual meeting
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-06 16:10:392025-06-03 16:11:4906/05/25 – ERA4TB will host public key-note speeches at annual meeting https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-06 08:00:332025-09-04 15:23:0706/05/25 – AMR Accelerator partner grit42 opened scientific data management platform
https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-05-06 08:00:332025-09-04 15:23:0706/05/25 – AMR Accelerator partner grit42 opened scientific data management platform https://amr-accelerator.eu/wp-content/uploads/2025/04/COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-28 14:46:392025-05-14 12:26:10Carina Sofia Matias: Setting the Standard for Infection Models
https://amr-accelerator.eu/wp-content/uploads/2025/04/COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-28 14:46:392025-05-14 12:26:10Carina Sofia Matias: Setting the Standard for Infection Models https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-16 12:03:232025-04-22 12:08:4216/04/25 – UNITE4TB Experts Featured in TB Management Video
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-16 12:03:232025-04-22 12:08:4216/04/25 – UNITE4TB Experts Featured in TB Management Video https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-10 16:08:312025-06-03 16:09:5210/04/25 – PrIMAVeRa Completes Mid-term Review
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-10 16:08:312025-06-03 16:09:5210/04/25 – PrIMAVeRa Completes Mid-term Review https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-09 13:45:272025-07-14 13:07:4509/04/25 – COMBINE pneumonia model at ESCMID Global – Vienna 2025
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-04-09 13:45:272025-07-14 13:07:4509/04/25 – COMBINE pneumonia model at ESCMID Global – Vienna 2025 https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-25 16:07:322025-06-03 16:08:1725/03/25 – PrIMAVeRa Partners Hold Modelling Workshop in Utrecht
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-25 16:07:322025-06-03 16:08:1725/03/25 – PrIMAVeRa Partners Hold Modelling Workshop in Utrecht https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-20 13:18:522025-03-25 13:20:0020/03/25 – UNITE4TB welcomes the ENABLE study to its growing clinical trial portfolio.
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-20 13:18:522025-03-25 13:20:0020/03/25 – UNITE4TB welcomes the ENABLE study to its growing clinical trial portfolio. https://amr-accelerator.eu/wp-content/uploads/2025/01/GNA-NOW-COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-19 12:09:162025-05-14 12:05:04Leonie von Berlin: Behind the Scenes of AMR Accelerator Data Management
https://amr-accelerator.eu/wp-content/uploads/2025/01/GNA-NOW-COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-19 12:09:162025-05-14 12:05:04Leonie von Berlin: Behind the Scenes of AMR Accelerator Data Management https://amr-accelerator.eu/wp-content/uploads/2024/11/Circle_RespiriTB-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-15 13:16:112025-03-25 13:18:1615/03/25 – RespiriTB Annual General Assembly in Vienna
https://amr-accelerator.eu/wp-content/uploads/2024/11/Circle_RespiriTB-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-15 13:16:112025-03-25 13:18:1615/03/25 – RespiriTB Annual General Assembly in Vienna https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_RespiriNTM-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-14 16:05:262025-06-03 16:06:2414/03/2025 – RespiriNTM Consortium Meets in Vienna
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_RespiriNTM-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-14 16:05:262025-06-03 16:06:2414/03/2025 – RespiriNTM Consortium Meets in Vienna https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-11 13:09:392025-03-25 13:11:3711/03/25 – PrIMAVeRa presents a video at ESCMID Global 2025
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-11 13:09:392025-03-25 13:11:3711/03/25 – PrIMAVeRa presents a video at ESCMID Global 2025 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-07 13:12:342025-03-25 13:15:0107/03/25 – Celebrating women in TB research
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-03-07 13:12:342025-03-25 13:15:0107/03/25 – Celebrating women in TB research https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-02-23 17:43:572025-09-25 19:17:3523/02/25 – COMBINE Publication on machine learning model to identify new antibiotics
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-02-23 17:43:572025-09-25 19:17:3523/02/25 – COMBINE Publication on machine learning model to identify new antibiotics https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_RespiriNTM-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-02-14 17:33:252025-02-14 17:33:2514/02/2025 – RespiriNTM 2024 Consortium Meetings Recap
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_RespiriNTM-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-02-14 17:33:252025-02-14 17:33:2514/02/2025 – RespiriNTM 2024 Consortium Meetings Recap
04/02/25 – PrIMAVeRa’s new publication on ‘Challenges in data sharing within the European community’
 https://amr-accelerator.eu/wp-content/uploads/2025/01/GNA-NOW-COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-01-23 16:21:022025-08-13 10:12:57Yojana Gadiya: AI and the Future of Antibiotic Discovery
https://amr-accelerator.eu/wp-content/uploads/2025/01/GNA-NOW-COMBINE-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-01-23 16:21:022025-08-13 10:12:57Yojana Gadiya: AI and the Future of Antibiotic Discovery https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-01-14 15:47:222025-01-23 15:48:5014/01/25 – Join UNITE4TB Young Investigators – Call for 2025 Members
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2025-01-14 15:47:222025-01-23 15:48:5014/01/25 – Join UNITE4TB Young Investigators – Call for 2025 Members https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-12 11:21:432025-01-13 11:28:0112/12/24 – ERA4TB researchers win prestigious Quality Innovation Award
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-12 11:21:432025-01-13 11:28:0112/12/24 – ERA4TB researchers win prestigious Quality Innovation Award https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-10 11:25:032025-01-13 11:26:3010/12/24 – PrIMAVeRa spotlighted in the latest AMR Accelerator Newsletter
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-10 11:25:032025-01-13 11:26:3010/12/24 – PrIMAVeRa spotlighted in the latest AMR Accelerator Newsletter https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-05 11:15:212025-01-13 11:26:5605/12/24 – UNITE4TB’s Journey: Interview with Project Lead, Derek Sloan
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-12-05 11:15:212025-01-13 11:26:5605/12/24 – UNITE4TB’s Journey: Interview with Project Lead, Derek Sloan https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-27 16:03:012025-01-23 16:04:5027/11/24 – UNITE4TB at Union Conference: Advancing TB Drug Development
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-27 16:03:012025-01-23 16:04:5027/11/24 – UNITE4TB at Union Conference: Advancing TB Drug Development https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-26 18:01:222025-05-12 15:04:50Nasreen Hassoun-Kheir: A Doctor’s Mission to Stop Superbugs
https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-26 18:01:222025-05-12 15:04:50Nasreen Hassoun-Kheir: A Doctor’s Mission to Stop Superbugs https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-22 15:33:592025-01-23 15:44:2222/11/24 – Research priorities for tackling AMR released during WAAW 2024
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-22 15:33:592025-01-23 15:44:2222/11/24 – Research priorities for tackling AMR released during WAAW 2024 https://amr-accelerator.eu/wp-content/uploads/2024/11/Circle_RespiriTB-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-22 11:06:102025-01-13 11:57:0122/11/24 – RespiriTB launches Phase 1 trial of long-acting injectable bedaquiline (BDQ LAI).
https://amr-accelerator.eu/wp-content/uploads/2024/11/Circle_RespiriTB-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-22 11:06:102025-01-13 11:57:0122/11/24 – RespiriTB launches Phase 1 trial of long-acting injectable bedaquiline (BDQ LAI). https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-21 14:41:582025-05-12 15:03:24Quentin J. Leclerc: Targeting Supercontactors for Safer Long-Term Care
https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-21 14:41:582025-05-12 15:03:24Quentin J. Leclerc: Targeting Supercontactors for Safer Long-Term Care https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-20 15:31:392025-01-23 15:33:2620/11/24 – PrIMAVeRa 3rd Annual Meeting, Seville, 07-08 Nov 2024
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-20 15:31:392025-01-23 15:33:2620/11/24 – PrIMAVeRa 3rd Annual Meeting, Seville, 07-08 Nov 2024 https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-18 16:01:372025-01-23 16:02:4518/11/24 – Advancing TB Treatment: An Update on the PARADIGM4TB Trial
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-18 16:01:372025-01-23 16:02:4518/11/24 – Advancing TB Treatment: An Update on the PARADIGM4TB Trial https://amr-accelerator.eu/wp-content/uploads/2025/11/All-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-12 11:20:372025-11-12 11:32:53Early Career Researchers Interview Series
https://amr-accelerator.eu/wp-content/uploads/2025/11/All-interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-12 11:20:372025-11-12 11:32:53Early Career Researchers Interview Series https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-06 15:27:262025-01-23 15:29:0706/11/24 – PrIMAVeRa Meeting with Vaccines Europe on AMR Reduction
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-11-06 15:27:262025-01-23 15:29:0706/11/24 – PrIMAVeRa Meeting with Vaccines Europe on AMR Reduction https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-29 17:49:502025-09-25 19:18:5629/10/24 – COMBINE ANNUAL MEETING 2024
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-29 17:49:502025-09-25 19:18:5629/10/24 – COMBINE ANNUAL MEETING 2024 https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-25 15:59:152025-01-23 16:00:4625/10/24 – Managing Adverse Events in TB Care: Key Insights UNITE4TB Webinar
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-25 15:59:152025-01-23 16:00:4625/10/24 – Managing Adverse Events in TB Care: Key Insights UNITE4TB Webinar
22/10/24 – BioVersys Joins RespiriNTM to Accelerate Development of the Broad-Spectrum Drug Candidates Against NTM Pulmonary Diseases
 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-22 15:11:322025-01-23 15:15:2722/10/24 – ERA4TB researchers win prestigious Quality Innovation Award
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-22 15:11:322025-01-23 15:15:2722/10/24 – ERA4TB researchers win prestigious Quality Innovation Award https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-14 15:58:182025-01-23 15:59:0614/10/24 – UNITE4TB Webinar: Intersecting the vulnerabilities of TB in migrants
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-14 15:58:182025-01-23 15:59:0614/10/24 – UNITE4TB Webinar: Intersecting the vulnerabilities of TB in migrants https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_GNANOW.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-02 11:54:472025-01-15 13:07:3102/10/24 – GNA NOW Annual & Expert Meeting
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_GNANOW.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-02 11:54:472025-01-15 13:07:3102/10/24 – GNA NOW Annual & Expert Meeting https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-01 11:51:162024-10-01 11:51:1625/09/24 – The COMBINE Project Coordinator, Anders Karlén is Pharmacist of the Year 2024
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-10-01 11:51:162024-10-01 11:51:1625/09/24 – The COMBINE Project Coordinator, Anders Karlén is Pharmacist of the Year 2024 https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-24 15:25:022025-11-05 11:02:1424/09/2024 Sustaining European Antibiotics Through R&D Funding
https://amr-accelerator.eu/wp-content/uploads/2024/11/PRIMAVERA_ECRI-banner.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-24 15:25:022025-11-05 11:02:1424/09/2024 Sustaining European Antibiotics Through R&D Funding https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-23 15:50:462025-01-23 15:57:5123/09/24 – Sustainable Funding Needed to Preserve EU AMR & Tuberculosis R&D
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-23 15:50:462025-01-23 15:57:5123/09/24 – Sustainable Funding Needed to Preserve EU AMR & Tuberculosis R&D
23/09/24 – Sustainable funding required to protect early phase antibiotics R&D for TB and beyond.

The AMR Accelerator calls to action: European capacity for antibiotic R&D requires long-term funding
 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-02 16:31:532024-09-02 18:12:2830/08/24 – ERA4TB to host workshop on TB Drug Development at The Union Conference 2024
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ERA4TB.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-02 16:31:532024-09-02 18:12:2830/08/24 – ERA4TB to host workshop on TB Drug Development at The Union Conference 2024 https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-02 16:28:032024-09-02 16:28:0322/08/24 – UNITE4TB webinar: Management of adverse events in TB care
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-09-02 16:28:032024-09-02 16:28:0322/08/24 – UNITE4TB webinar: Management of adverse events in TB care
13/08/24 – PrIMAVeRa project publishes on contact network dynamics to prevent pathogen transmission in hospitals
 https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-07-10 13:48:312024-07-10 13:50:0609/07/24 – UNITE4TB Completes Recruitment for DECISION Trial
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-07-10 13:48:312024-07-10 13:50:0609/07/24 – UNITE4TB Completes Recruitment for DECISION Trial https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-07-09 09:35:352024-07-09 09:35:3525/06/24 – Key insights from PrIMAVeRa project year two progress review meeting
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-07-09 09:35:352024-07-09 09:35:3525/06/24 – Key insights from PrIMAVeRa project year two progress review meeting https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-06-12 19:42:372024-07-10 13:54:38UNITE4TB Newsletter – June 2024
https://amr-accelerator.eu/wp-content/uploads/2021/10/unite4tb-4.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-06-12 19:42:372024-07-10 13:54:38UNITE4TB Newsletter – June 2024 https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-06-03 12:34:312024-07-10 13:54:22AMR Accelerator Newsletter – June 2024
https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-06-03 12:34:312024-07-10 13:54:22AMR Accelerator Newsletter – June 2024 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ABDirect.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-05-28 12:42:522025-10-08 10:19:0728/05/24 – AB-Direct reaches its end
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_ABDirect.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-05-28 12:42:522025-10-08 10:19:0728/05/24 – AB-Direct reaches its end https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-05-21 14:14:302024-05-28 12:47:3115/05/24 – PrIMAVeRa project partners present posters at ECCMID 2024
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-05-21 14:14:302024-05-28 12:47:3115/05/24 – PrIMAVeRa project partners present posters at ECCMID 2024 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-04-11 16:21:502024-05-28 12:50:5111/04/24 – RECORDING OF WEBINAR ON TPP FOR ANTIBIOTICS
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_COMBINE.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-04-11 16:21:502024-05-28 12:50:5111/04/24 – RECORDING OF WEBINAR ON TPP FOR ANTIBIOTICS https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-04-09 09:58:352024-05-28 13:27:2109/04/24 – AMR ACCELERATOR CROSS-PROJECT MEETING 2024
https://amr-accelerator.eu/wp-content/uploads/2024/04/Asset-1.png
218
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-04-09 09:58:352024-05-28 13:27:2109/04/24 – AMR ACCELERATOR CROSS-PROJECT MEETING 2024 https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-03-15 10:47:112024-05-28 13:25:2108/03/24 – PrIMAVeRa participates in AMR Accelerator Cross-Project Meeting 2024
https://amr-accelerator.eu/wp-content/uploads/2022/02/primavera-1.png
217
230
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-03-15 10:47:112024-05-28 13:25:2108/03/24 – PrIMAVeRa participates in AMR Accelerator Cross-Project Meeting 2024 https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_TricTB.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-03-05 09:00:582024-05-28 13:32:5405/03/24 – TRIC-TB reaches its end
https://amr-accelerator.eu/wp-content/uploads/2020/09/Circle_TricTB.png
217
229
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-03-05 09:00:582024-05-28 13:32:5405/03/24 – TRIC-TB reaches its end https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-02-15 13:11:242024-02-15 13:11:2412/02/24 – ERA4TB researcher wins award for innovative research design
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-02-15 13:11:242024-02-15 13:11:2412/02/24 – ERA4TB researcher wins award for innovative research design
25/01/24 – Public launch of TB-Platform for the Aggregation of Preclinical Experiments Data (TB-APEX)

23/01/24 – GNA NOW consortium expands its scope to support the under-studied condition of severe diarrhoea
 https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-01-17 09:07:162024-01-23 09:08:0117/01/24 – ERA4TB launches new project video on progress
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2024-01-17 09:07:162024-01-23 09:08:0117/01/24 – ERA4TB launches new project video on progress
12/12/23 – BioVersys receives U.S. FDA orphan-drug designation for alpibectir and ethionamide fixed-dose combination for treatment of tuberculosis
 https://amr-accelerator.eu/wp-content/uploads/2023/11/Respiris_interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-30 11:24:412024-12-13 12:01:05Meindert Lamers: RespiriTB & RespiriNTM Projects
https://amr-accelerator.eu/wp-content/uploads/2023/11/Respiris_interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-30 11:24:412024-12-13 12:01:05Meindert Lamers: RespiriTB & RespiriNTM Projects
24/11/23 – PrIMAVeRa provides in-depth reports on current evidence base of burden of AMR in Europe

24/11/23 – PrIMAVeRa 2nd annual meeting successfully held in hybrid format in Utrecht on 15-16 November 2023
 https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-22 14:24:342023-11-22 14:24:3422/11/23 – UNITE4TB/PAN-TB Joint Satellite Session at the 2023 Union Conference
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-22 14:24:342023-11-22 14:24:3422/11/23 – UNITE4TB/PAN-TB Joint Satellite Session at the 2023 Union Conference https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-14 17:47:582023-11-15 09:08:0514/11/23 – Collaboration to improve the quality of in vivo antibiotics testing
https://amr-accelerator.eu/wp-content/uploads/2023/11/AMR-Accelerator_square-3-e1716812644571.png
200
250
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-11-14 17:47:582023-11-15 09:08:0514/11/23 – Collaboration to improve the quality of in vivo antibiotics testing
08/11/23 – A new dawn in the fight against Tuberculosis: UNITE4TB announces start of clinical trials
 https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-06-22 14:13:042023-06-27 14:14:0622/06/23 – More than 100 ERA4TB members gather for 7th Consortium Meeting
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-06-22 14:13:042023-06-27 14:14:0622/06/23 – More than 100 ERA4TB members gather for 7th Consortium Meeting https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-06-19 15:14:592023-09-14 15:15:1119/06/23 – PrIMAVeRa Project Appoints Scientific Advisory Committee
https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-06-19 15:14:592023-09-14 15:15:1119/06/23 – PrIMAVeRa Project Appoints Scientific Advisory Committee https://amr-accelerator.eu/wp-content/uploads/2021/03/IMI-AMR-Accelerator_Slogan_square_RGB.png
1024
1339
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-11 15:14:312023-05-17 14:53:2511/05/23 – VOICES FROM THE AMR ACCELERATOR
https://amr-accelerator.eu/wp-content/uploads/2021/03/IMI-AMR-Accelerator_Slogan_square_RGB.png
1024
1339
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-11 15:14:312023-05-17 14:53:2511/05/23 – VOICES FROM THE AMR ACCELERATOR https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-10 14:32:542023-05-17 14:34:1010/05/23 – Impressions of the 2023 UNITE4TB Annual Meeting & Symposium
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-10 14:32:542023-05-17 14:34:1010/05/23 – Impressions of the 2023 UNITE4TB Annual Meeting & Symposium https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-03 11:52:382023-07-06 11:53:5003/05/23 – A look back at the 2023 UNITE4TB Annual Consortium Meeting and Symposium
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-05-03 11:52:382023-07-06 11:53:5003/05/23 – A look back at the 2023 UNITE4TB Annual Consortium Meeting and Symposium https://amr-accelerator.eu/wp-content/uploads/2021/03/IMI-AMR-Accelerator_Slogan_square_RGB.png
1024
1339
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-04-06 08:08:562023-04-13 16:51:1306/04/23 – PUTTING AMR ACCELERATOR SCIENCE ON THE COMMUNITY AGENDA
https://amr-accelerator.eu/wp-content/uploads/2021/03/IMI-AMR-Accelerator_Slogan_square_RGB.png
1024
1339
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-04-06 08:08:562023-04-13 16:51:1306/04/23 – PUTTING AMR ACCELERATOR SCIENCE ON THE COMMUNITY AGENDA https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-03-24 14:53:412023-04-12 13:40:3724/03/23 – UNITE4TB partakes in World TB Day 2023: Yes! We can end TB!
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-03-24 14:53:412023-04-12 13:40:3724/03/23 – UNITE4TB partakes in World TB Day 2023: Yes! We can end TB! https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-03-21 14:49:102023-04-20 10:33:2221/03/23 – ERA4TB members attend key meetings on Antimicrobial Resistance in Basel
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-03-21 14:49:102023-04-20 10:33:2221/03/23 – ERA4TB members attend key meetings on Antimicrobial Resistance in Basel
16/02/23 – ERA4TB to partner with UNITE4TB for webinar focusing on community engagement in TB research
 https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-31 15:23:422023-02-17 15:23:5331/01/23 – BioVersys announces first patient dosed in Phase 2a clinical trial with BVL-GSK098
https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-31 15:23:422023-02-17 15:23:5331/01/23 – BioVersys announces first patient dosed in Phase 2a clinical trial with BVL-GSK098 https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-31 14:53:542023-02-17 15:01:5131/01/23 – Apply now to join the UNITE4TB Young Investigators Group
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-31 14:53:542023-02-17 15:01:5131/01/23 – Apply now to join the UNITE4TB Young Investigators Group https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-30 15:12:302023-02-17 15:13:0930/01/23 – ERA4TB partners gather for a face-to-face event in Paris for 6th Consortium Meeting
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-30 15:12:302023-02-17 15:13:0930/01/23 – ERA4TB partners gather for a face-to-face event in Paris for 6th Consortium Meeting https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-23 15:14:572023-02-17 15:17:4623/01/23 – ERA4TB marks its halfway point with 6th Consortium meeting in Paris
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2023-01-23 15:14:572023-02-17 15:17:4623/01/23 – ERA4TB marks its halfway point with 6th Consortium meeting in Paris https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-12-14 14:52:232023-02-17 15:16:4114/12/22 – UNITE4TB 2022 highlights
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-12-14 14:52:232023-02-17 15:16:4114/12/22 – UNITE4TB 2022 highlights
12/12/22 – The power of words: the importance of appropriate language use in TB communications from UNITE4TB
 https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-11-30 15:00:272023-02-17 15:01:2530/11/22 – PPrIMAVeRa 1st annual meeting successfully held in a hybrid event in Verona
https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-11-30 15:00:272023-02-17 15:01:2530/11/22 – PPrIMAVeRa 1st annual meeting successfully held in a hybrid event in Verona
Interview with Hugh Laverty: Executive Director ad interim at Innovative Health Initiative

16/11/22 – Meaningful engagement of the community in TB Research & Development: Best practice initiative from UNITE4TB

25/10/22 – New publication from GNA NOW Consortium published by the American Society for Microbiology

17/10/22 – ERA4TB Webinar on Challenges of tuberculosis (TB) drug development and access – clinical, regulatory and HTA perspectives
 https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-21 08:14:132023-04-20 10:33:0121/09/22 – ERA4TB participates in the #WorldEBHCDay campaign, ‘Partnerships for Purpose’
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-21 08:14:132023-04-20 10:33:0121/09/22 – ERA4TB participates in the #WorldEBHCDay campaign, ‘Partnerships for Purpose’ https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:21:342023-04-20 10:39:1119/09/22 – UNITE4TB Clinical trial preparations in Vietnam
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:21:342023-04-20 10:39:1119/09/22 – UNITE4TB Clinical trial preparations in Vietnam https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:21:022023-04-20 10:39:3219/09/22 – UNITE4TB Exploring synergies and collaborations
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:21:022023-04-20 10:39:3219/09/22 – UNITE4TB Exploring synergies and collaborations https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:20:082023-04-20 10:40:2219/09/22 – The UNITE4TB Community Advisory Group: Voice of the TB community
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:20:082023-04-20 10:40:2219/09/22 – The UNITE4TB Community Advisory Group: Voice of the TB community https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:19:262023-04-20 10:31:1019/09/22 – Year-1 highlights from the UNITE4TB project leads
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-09-19 12:19:262023-04-20 10:31:1019/09/22 – Year-1 highlights from the UNITE4TB project leads https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-06-21 15:12:252022-06-21 15:12:2521/06/22 – BioVersys successfully completes Phase I clinical trials of BVL-GSK098
https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-06-21 15:12:252022-06-21 15:12:2521/06/22 – BioVersys successfully completes Phase I clinical trials of BVL-GSK098
08/06/22 – Positive results for preclinical development of the first-in-class antibiotics NOSO-502
 https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-18 08:00:222023-04-20 10:35:5218/05/22 – UNITE4TB 2022 Annual Meeting
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-18 08:00:222023-04-20 10:35:5218/05/22 – UNITE4TB 2022 Annual Meeting https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-04 08:44:352022-07-28 08:46:2304/05/22 – Expert interview: Microbiologists on tuberculosis diagnostics
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-04 08:44:352022-07-28 08:46:2304/05/22 – Expert interview: Microbiologists on tuberculosis diagnostics https://amr-accelerator.eu/wp-content/uploads/2022/06/PRIMAVERA_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-01 13:20:552025-11-12 14:14:43Interview with Irina Meln: PrIMAVeRa Project Coordinator
https://amr-accelerator.eu/wp-content/uploads/2022/06/PRIMAVERA_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-05-01 13:20:552025-11-12 14:14:43Interview with Irina Meln: PrIMAVeRa Project Coordinator https://amr-accelerator.eu/wp-content/uploads/2022/06/Accelerator-1-scaled_web.png
750
1000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-04-06 14:18:062023-04-20 10:36:1606/04/22 – 3rd AMR Accelerator cross-project meeting held as in-person event
https://amr-accelerator.eu/wp-content/uploads/2022/06/Accelerator-1-scaled_web.png
750
1000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-04-06 14:18:062023-04-20 10:36:1606/04/22 – 3rd AMR Accelerator cross-project meeting held as in-person event https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-02-25 15:07:352023-04-20 10:27:1225/02/22 – UNITE4TB Community Advisory Group holds inaugural meeting
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-02-25 15:07:352023-04-20 10:27:1225/02/22 – UNITE4TB Community Advisory Group holds inaugural meeting https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-02-03 11:44:112023-04-20 10:27:4803/02/22 – PrIMAVeRa WP2 workshop, virtually held on 27-28 January 2022
https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2022-02-03 11:44:112023-04-20 10:27:4803/02/22 – PrIMAVeRa WP2 workshop, virtually held on 27-28 January 2022 https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-12-03 16:18:362023-04-20 10:28:4103/12/21 – ERA4TB schedules the 4th consortium meeting on 27th and 28th of January 2022
https://amr-accelerator.eu/wp-content/uploads/2021/12/ERA4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-12-03 16:18:362023-04-20 10:28:4103/12/21 – ERA4TB schedules the 4th consortium meeting on 27th and 28th of January 2022 https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-12-01 13:52:072023-04-20 10:30:2701/12/21 – PrIMAVeRa kick-off meeting successfully marks the official project launch
https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-12-01 13:52:072023-04-20 10:30:2701/12/21 – PrIMAVeRa kick-off meeting successfully marks the official project launch https://amr-accelerator.eu/wp-content/uploads/2021/11/IMI_ERA4TB.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-23 14:05:212025-11-12 14:15:43Interview with Almari Conradie: Director of Clinical Operations at TB Alliance
https://amr-accelerator.eu/wp-content/uploads/2021/11/IMI_ERA4TB.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-23 14:05:212025-11-12 14:15:43Interview with Almari Conradie: Director of Clinical Operations at TB Alliance https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-22 11:34:522023-04-20 10:30:0022/11/21 – Antimicrobial Resistance: the silent pandemic
https://amr-accelerator.eu/wp-content/uploads/2021/12/PRIMAVERA_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-22 11:34:522023-04-20 10:30:0022/11/21 – Antimicrobial Resistance: the silent pandemic
18/11/21 – Tuberculosis projects represent the biggest ever funding effort to combat the disease
 https://amr-accelerator.eu/wp-content/uploads/2021/04/UNITE4TB_Interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-01 13:50:212025-11-12 14:17:36Interview with Martin Boeree: UNITE4TB Project Coordinator
https://amr-accelerator.eu/wp-content/uploads/2021/04/UNITE4TB_Interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-01 13:50:212025-11-12 14:17:36Interview with Martin Boeree: UNITE4TB Project Coordinator https://amr-accelerator.eu/wp-content/uploads/2022/03/IMI_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-01 13:38:172025-11-12 14:17:19Interview with Pierre Meulien: Executive Director at Innovative Medicines Initiative
https://amr-accelerator.eu/wp-content/uploads/2022/03/IMI_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-11-01 13:38:172025-11-12 14:17:19Interview with Pierre Meulien: Executive Director at Innovative Medicines Initiative https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-07-15 19:20:102021-12-16 09:04:2415/07/21 – Launch of UNITE4TB partnership
https://amr-accelerator.eu/wp-content/uploads/2021/12/UNITE4TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-07-15 19:20:102021-12-16 09:04:2415/07/21 – Launch of UNITE4TB partnership https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-07-06 11:25:392023-04-20 10:29:1606/07/21 – BioVersys pipeline presentations at ECCMID July 9-12, 2021
https://amr-accelerator.eu/wp-content/uploads/2021/12/TRiC_TB_News_5000x4000px.png
4000
5000
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-07-06 11:25:392023-04-20 10:29:1606/07/21 – BioVersys pipeline presentations at ECCMID July 9-12, 2021 https://amr-accelerator.eu/wp-content/uploads/2021/05/gna-now-open-call-2.png
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-05-10 11:40:412021-07-15 20:08:0910/05/21 – Open Call: Apply now to join the GNA NOW Consortium
https://amr-accelerator.eu/wp-content/uploads/2021/05/gna-now-open-call-2.png
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-05-10 11:40:412021-07-15 20:08:0910/05/21 – Open Call: Apply now to join the GNA NOW Consortium https://amr-accelerator.eu/wp-content/uploads/2022/12/COMBINE_Interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-05-09 09:43:282025-11-12 14:18:21Interview with Anders Karlén: COMBINE Project Coordinator, Uppsala University
https://amr-accelerator.eu/wp-content/uploads/2022/12/COMBINE_Interview.png
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-05-09 09:43:282025-11-12 14:18:21Interview with Anders Karlén: COMBINE Project Coordinator, Uppsala University https://amr-accelerator.eu/wp-content/uploads/2021/04/GNA-NOW_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-04-15 10:09:392025-11-12 14:19:55Interview with Eric Bacqué: GNA NOW Project Lead, Evotec
https://amr-accelerator.eu/wp-content/uploads/2021/04/GNA-NOW_Interview.jpg
250
800
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-04-15 10:09:392025-11-12 14:19:55Interview with Eric Bacqué: GNA NOW Project Lead, Evotec https://amr-accelerator.eu/wp-content/uploads/2021/03/amr-accelerator-logo.png
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-03-25 11:53:282021-07-15 20:08:2825/03/21 – Tackling antibiotic resistance together
https://amr-accelerator.eu/wp-content/uploads/2021/03/amr-accelerator-logo.png
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-03-25 11:53:282021-07-15 20:08:2825/03/21 – Tackling antibiotic resistance together https://amr-accelerator.eu/wp-content/uploads/2022/12/AB-Direct-e1716892977411.png
200
600
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-02-01 11:42:352023-04-20 10:32:1802/21 -Launch of AB-Direct Phase 1 study
https://amr-accelerator.eu/wp-content/uploads/2022/12/AB-Direct-e1716892977411.png
200
600
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-02-01 11:42:352023-04-20 10:32:1802/21 -Launch of AB-Direct Phase 1 study https://amr-accelerator.eu/wp-content/uploads/2021/01/TRIC-TB_Logo.jpg
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-01-12 14:26:332021-07-15 20:08:4912/01/21 – BioVersys Announces First Subjects Dosed in Phase 1 Clinical Trial of BVL-GSK098
https://amr-accelerator.eu/wp-content/uploads/2021/01/TRIC-TB_Logo.jpg
300
455
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2021-01-12 14:26:332021-07-15 20:08:4912/01/21 – BioVersys Announces First Subjects Dosed in Phase 1 Clinical Trial of BVL-GSK098 https://amr-accelerator.eu/wp-content/uploads/2020/07/Annual-meeting-image.png
1440
2560
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-06-24 12:10:182023-04-20 10:24:5324/06/20 – Successful First Annual Meeting for IMI project GNA NOW
https://amr-accelerator.eu/wp-content/uploads/2020/07/Annual-meeting-image.png
1440
2560
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-06-24 12:10:182023-04-20 10:24:5324/06/20 – Successful First Annual Meeting for IMI project GNA NOW https://amr-accelerator.eu/wp-content/uploads/2020/07/Tric-TB_logo.png
285
489
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-06-23 14:43:012023-04-20 10:23:1423/06/20 – BioVersys receives QIDP designation for BVL-GSK098 and ETH
https://amr-accelerator.eu/wp-content/uploads/2020/07/Tric-TB_logo.png
285
489
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-06-23 14:43:012023-04-20 10:23:1423/06/20 – BioVersys receives QIDP designation for BVL-GSK098 and ETH https://amr-accelerator.eu/wp-content/uploads/2019/05/COMBINE_logo-e1647497540928.jpg
435
1360
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-03-11 14:12:092022-03-17 07:23:5811/03/20 – COMBINE Call for preclinical and clinical data sets
https://amr-accelerator.eu/wp-content/uploads/2019/05/COMBINE_logo-e1647497540928.jpg
435
1360
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-03-11 14:12:092022-03-17 07:23:5811/03/20 – COMBINE Call for preclinical and clinical data sets https://amr-accelerator.eu/wp-content/uploads/2020/01/ERA4TB_logotipos_hq-2.png
150
680
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-01-29 16:29:512021-07-15 20:11:3629/01/20 – ERA4TB, the new IMI project to accelerate the fight against tuberculosis
https://amr-accelerator.eu/wp-content/uploads/2020/01/ERA4TB_logotipos_hq-2.png
150
680
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2020-01-29 16:29:512021-07-15 20:11:3629/01/20 – ERA4TB, the new IMI project to accelerate the fight against tuberculosis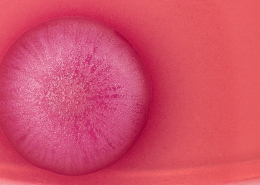 © Daniela Beckmann
https://amr-accelerator.eu/wp-content/uploads/2019/05/IMG_9931_edit_Ausschnitt-1-e1580309178365.jpg
989
1840
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2019-12-03 10:00:222021-07-15 20:11:5903/12/19 – IMI project COMBINE launched
© Daniela Beckmann
https://amr-accelerator.eu/wp-content/uploads/2019/05/IMG_9931_edit_Ausschnitt-1-e1580309178365.jpg
989
1840
m.reich
/wp-content/uploads/2024/08/IMI-AMR-Accelerator_Slogan_quer_RGB.png
m.reich2019-12-03 10:00:222021-07-15 20:11:5903/12/19 – IMI project COMBINE launchedLearn more about the AMR Accelerator Projects
Link to: AB-Direct
- 7 participants
- > 3.7 M€ budget
- 4 years & 3 months. Started 1 July 2019
Antibiotic distribution and recovery in tissue

Link to: COMBINE
- 11 participants
- 25 M€ budget
- 6 years. Started 1 Nov 2019
Coordination and support across the AMR Accelerator. Research to strengthen the scientific basis in the AMR field.

Link to: ERA4TB
- 32 participants
- > 207 M€ budget
- 6 years. Started 1 Jan 2020
Development of anti-TB drug combinations

Link to: GNA NOW
- 12 participants
- 21.6 M€ budget
- 6 years. Started 1 July 2019
New antibiotics to treat Gram-negative infections

Link to: PrIMAVeRa
- 18 participants
- 9.25 M€ budget
- 5 years. Started 1 November 2021
Predicting the Impact of Monoclonal Antibodies & Vaccines on Antimicrobial Resistance

Link to: RespiriNTM
- 9 participants
- > 8 M€ budget
- 6 years. Started 1 May 2019
Novel assets for non-tuberculous mycobacteria

Link to: RespiriTB
- 9 participants
- > 9.9 M€ budget
- 6 years. Started 1 May 2019
New assets for multidrug-resistant tuberculosis

Link to: TRIC-TB
- 2 participants
- > 8.3 M€ budget
- 4 years & 10 months. Started 1 May 2019
Defining a new place for Ethionamide in 1st-line TB treatments






Follow us:
| The AMR Accelerator has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreements No 853967 I 853989 I 853979 I 853932 I 853800 I 853903 I 853976 I 101007873 I 101034420. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. ERA4TB receives additional support from Global Alliance for TB Drug Development, Bill & Melinda Gates Foundation and University of Dundee. UNITE4TB receives additional support from Deutsches Zentrum für Infektionsforschung e. V. (DZIF), and Ludwig-Maximilians-Universität München (LMU). EFPIA/AP contribute to 50% of funding, whereas the contribution of DZIF and the LMU University Hospital Munich has been granted by the German Federal Ministry of Education and Research. https://www.imi.europa.eu/ |





